処分場の緩衝材間隙水の酸化還元電位へのオーバーパック腐食の影響; 重要パラメータの取得及び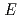 hの予備解析(受託研究)
hの予備解析(受託研究)
Effects of overpack corrosion on redox potential of bentonite pore water under geological disposal environment; Important parameter acquisition and a preliminary 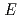 h analysis (Contract research)
h analysis (Contract research)
大塚 伊知郎; 瀧 洋*; 山口 徹治  ; 飯田 芳久
; 飯田 芳久  ; 山田 文香; 稲田 大介*; 田中 忠夫
; 山田 文香; 稲田 大介*; 田中 忠夫 
Otsuka, Ichiro; Taki, Hiroshi*; Yamaguchi, Tetsuji; Iida, Yoshihisa; Yamada, Fumika; Inada, Daisuke*; Tanaka, Tadao
高レベル放射性廃棄物処分場において、緩衝材の間隙に含まれる水(緩衝材間隙水)の酸化還元状態は、放射性核種の化学的性質に影響するため、重要な評価因子である。炭素鋼オーバーパックの腐食が緩衝材間隙水の酸化還元電位(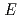 h)に与える影響を評価するうえで重要な腐食生成物の熱力学データ,炭素鋼の腐食速度を文献調査及び実験により取得し、カソード反応の定量評価を行った。また、地球化学計算コードPhreeq Cで予察的な解析を行い、詳細な解析を行ううえでの課題を抽出した。結果は以下のようにまとめられる。(1)Fe
h)に与える影響を評価するうえで重要な腐食生成物の熱力学データ,炭素鋼の腐食速度を文献調査及び実験により取得し、カソード反応の定量評価を行った。また、地球化学計算コードPhreeq Cで予察的な解析を行い、詳細な解析を行ううえでの課題を抽出した。結果は以下のようにまとめられる。(1)Fe , FeOH
, FeOH , Fe(OH)
, Fe(OH) (aq), Fe(OH)
(aq), Fe(OH)
 , Fe(OH)
, Fe(OH)
 , Fe
, Fe , FeS
, FeS , FeCO
, FeCO ,Fe(OH)
,Fe(OH) (s), Fe
(s), Fe O
O , Fe
, Fe CO
CO (OH)
(OH) , Fe(cr)の熱力学データの最確値及び誤差を文献調査及び実験により取得した。(2)炭素鋼の腐食速度をpHと硫化物イオン濃度の関数として定式化した。(3)ガス蓄積型腐食試験からカソード反応は水素発生反応が支配的であることがわかった。(4)予察的な
, Fe(cr)の熱力学データの最確値及び誤差を文献調査及び実験により取得した。(2)炭素鋼の腐食速度をpHと硫化物イオン濃度の関数として定式化した。(3)ガス蓄積型腐食試験からカソード反応は水素発生反応が支配的であることがわかった。(4)予察的な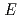 h評価解析から、1000年後の
h評価解析から、1000年後の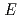 hは、約-600mV又は750mVを得たので、CH
hは、約-600mV又は750mVを得たので、CH (aq)/CO
(aq)/CO
 もしくはH
もしくはH (aq)/H
(aq)/H Oに支配されると考えられる。
Oに支配されると考えられる。
The influence of carbon steel overpack corrosion on redox potential (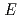 h) of bentonite pore water under geological disposal environment was investigated. The thermodynamics data of corrosion products, the corrosion rate of carbon steel, and the information on cathode reactions were acquired by experiments and literature survey. We conducted preliminary analysis of
h) of bentonite pore water under geological disposal environment was investigated. The thermodynamics data of corrosion products, the corrosion rate of carbon steel, and the information on cathode reactions were acquired by experiments and literature survey. We conducted preliminary analysis of 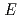 h, ascertained the validity of Phreeq C and identified important points on the analysis. Results were summarized as follows. (1) Thermodynamic data of Fe
h, ascertained the validity of Phreeq C and identified important points on the analysis. Results were summarized as follows. (1) Thermodynamic data of Fe , FeOH
, FeOH , Fe(OH)
, Fe(OH) (aq), Fe(OH)
(aq), Fe(OH)
 , Fe(OH)
, Fe(OH)
 , Fe
, Fe , FeS
, FeS (pyrite), FeCO
(pyrite), FeCO (siderite),Fe(OH)
(siderite),Fe(OH) (s), Fe
(s), Fe O
O (magnetite), Fe(cr) were determined by literature survey. The solubility product of Fe
(magnetite), Fe(cr) were determined by literature survey. The solubility product of Fe CO
CO (OH)
(OH) was determined experimentally, and thermodynamic data were estimated. (2) The corrosion rate of carbon steel was obtained as a function of pH and sulfide ion concentration. (3) After corrosion tests of carbon steel, no CH
was determined experimentally, and thermodynamic data were estimated. (2) The corrosion rate of carbon steel was obtained as a function of pH and sulfide ion concentration. (3) After corrosion tests of carbon steel, no CH , HS
, HS and H
and H S, the reduction product of CO
S, the reduction product of CO
 and SO
and SO
 ,were not detected in liquid and gas phases. (4) Preliminary analysis showed that the redox couple changed as HS
,were not detected in liquid and gas phases. (4) Preliminary analysis showed that the redox couple changed as HS (aq)/SO
(aq)/SO
 , CH
, CH (aq)/CO
(aq)/CO
 , H
, H (aq)/H
(aq)/H O during the evaluation period. After 1000 years,
O during the evaluation period. After 1000 years, 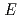 h attained about -500 to -600 mV (vs. NHE) or -750 mV controlled by CH
h attained about -500 to -600 mV (vs. NHE) or -750 mV controlled by CH (aq)/CO
(aq)/CO
 ,or H
,or H (aq)/H
(aq)/H O, respectively.
O, respectively.