Study on the relation between the crystal structure and thermal stability of FeUO and CrUO
and CrUO
FeUO およびCrUO
およびCrUO の結晶構造と熱的安定性に関する研究
の結晶構造と熱的安定性に関する研究
秋山 大輔*; 日下 良二 
 ; 熊谷 友多
; 熊谷 友多 

 ; 中田 正美
; 中田 正美 
 ; 渡邉 雅之
; 渡邉 雅之 

 ; 岡本 芳浩
; 岡本 芳浩 
 ; 永井 崇之
; 永井 崇之 
 ; 佐藤 修彰*; 桐島 陽*
; 佐藤 修彰*; 桐島 陽*
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
ウラン酸鉄,ウラン酸クロム、およびその固溶体を合成し、これらのウラン酸塩が異なる熱的安定性を示すメカニズムを研究した。熱的安定性を評価するため、ウラン酸塩試料の熱重量分析を実施した結果、ウラン酸クロムの分解温度(約1250 C)に対してウラン酸鉄は低温(約800
C)に対してウラン酸鉄は低温(約800 C)で分解するが、クロムを含む固溶体では熱分解に対する安定性が高まることが分かった。この熱的安定性と結晶構造との関係性を調べるため、エックス線結晶構造解析,エックス線吸収微細構造測定,メスバウアー分光測定,ラマン分光分析による詳細な結晶構造と物性の評価を行ったが、本研究で用いたウラン酸塩試料の間に明瞭な差異は観測されなかった。そのため、熱的安定性の違いは結晶構造に起因するものではなく、鉄とクロムとの酸化還元特性の違いによるものと推定した。クロムは3価が極めて安定であるのに対して、鉄の原子価は2価と3価を取ることができる。このため、ウラン酸鉄の場合には結晶中でウランと鉄との酸化還元反応が起こり、低温での分解反応を誘起したものと考えられる。
C)で分解するが、クロムを含む固溶体では熱分解に対する安定性が高まることが分かった。この熱的安定性と結晶構造との関係性を調べるため、エックス線結晶構造解析,エックス線吸収微細構造測定,メスバウアー分光測定,ラマン分光分析による詳細な結晶構造と物性の評価を行ったが、本研究で用いたウラン酸塩試料の間に明瞭な差異は観測されなかった。そのため、熱的安定性の違いは結晶構造に起因するものではなく、鉄とクロムとの酸化還元特性の違いによるものと推定した。クロムは3価が極めて安定であるのに対して、鉄の原子価は2価と3価を取ることができる。このため、ウラン酸鉄の場合には結晶中でウランと鉄との酸化還元反応が起こり、低温での分解反応を誘起したものと考えられる。
FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U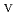
 Fe
Fe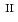
 U
U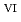 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.