Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nishioka, Shunichiro; Nakajima, Kunihisa; Suzuki, Eriko; Osaka, Masahiko
Journal of Nuclear Science and Technology, 56(11), p.988 - 995, 2019/11
Times Cited Count:12 Percentile:79.16(Nuclear Science & Technology)In order to contribute to improvement of Cs chemisorption model used in severe accident analysis codes, the influence of chemical factors (temperature, atmosphere, concentration of affecting chemical elements etc.) on the Cs chemisorption behaviour onto stainless steel was investigated experimentally. It was found that the surface reaction rate constant used in the current Cs-chemisorption model was influenced by not only temperature, as already known, but also atmosphere, cesium hydroxide (CsOH) concentration in the gas phase and silicon content in SS304. Such chemical factors should be considered for the construction of the improved Cs-chemisorption model. Another important finding is that the chemisorption behavior at lower temperatures, around 873 K, could differ from those above 1073 K. Namely, Cs-Fe-O compounds would form as the main Cs-chemisorbed compounds at 873 K while Cs-Si-Fe-O compounds at more than 1073 K.
Miyahara, Naoya; Miwa, Shuhei; Horiguchi, Naoki; Sato, Isamu*; Osaka, Masahiko
Journal of Nuclear Science and Technology, 56(2), p.228 - 240, 2019/02
Times Cited Count:7 Percentile:60.64(Nuclear Science & Technology)In order to improve LWR source term under severe accident conditions, the first version of a fission product (FP) chemistry database named "ECUME" was developed. The ECUME is intended to include major chemical reactions and their effective kinetic constants for representative SA sequences. It is expected that the ECUME can serve as a fundamental basis from which FP chemical models in the SA analysis codes can be elaborated. The implemented chemical reactions in the first version were those for representative gas species in Cs-I-B-Mo-O-H system. The chemical reaction kinetic constants were evaluated from either literature data or calculated values using ab-initio calculations. The sample chemical reaction calculation using the presently constructed dataset showed meaningful kinetics effects at 1000 K. Comparison of the chemical equilibrium compositions by using the dataset with those by chemical equilibrium calculations has shown rather good consistency for the representative Cs-I-B-Mo-O-H species. From these results, it was concluded that the present dataset should be useful to evaluate FP chemistry in Cs-I-B-Mo-O-H system under LWA SA conditions.
Mukai, Satoru*; Umehara, Ryuji*; Hanawa, Satoshi; Kasahara, Shigeki; Nishiyama, Yutaka
Proceedings of 20th International Conference on Water Chemistry of Nuclear Reactor Systems (NPC 2016) (USB Flash Drive), 9 Pages, 2016/10
In Japanese PWR, the concentration of dissolved hydrogen in the primary coolant is controlled in the range from 25 cc/kg-H O to 35 cc/kg-H
O to 35 cc/kg-H O for suppression of water decomposition. However this concentration is desired to reduce for the purpose of radiation source reduction in Japan. So, the concentration due to water radiolysis in primary coolant was evaluated at lower hydrogen concentration by the water radiolysis model in consideration of
O for suppression of water decomposition. However this concentration is desired to reduce for the purpose of radiation source reduction in Japan. So, the concentration due to water radiolysis in primary coolant was evaluated at lower hydrogen concentration by the water radiolysis model in consideration of  ray, fast neutron and alpha ray due to the reaction
ray, fast neutron and alpha ray due to the reaction  B(n,
B(n, )
) Li. The results of evaluation showed that the water radiolysis was suppressed even if the hydrogen concentration was decreased to 5 cc/kg-H
Li. The results of evaluation showed that the water radiolysis was suppressed even if the hydrogen concentration was decreased to 5 cc/kg-H O. The effects of the different G-value and the rate constants of major reaction on the concentration of H
O. The effects of the different G-value and the rate constants of major reaction on the concentration of H O
O and O
and O were studied under hydrogen addition. We also focused on the effect of the alpha radiolysis in boron acid water.
were studied under hydrogen addition. We also focused on the effect of the alpha radiolysis in boron acid water.
Iwai, Yasunori
Fusion Engineering and Design, 98-99, p.1796 - 1799, 2015/10
Times Cited Count:5 Percentile:39.5(Nuclear Science & Technology)Hydrophobic platinum catalysts have been widely applied in the field of nuclear fusion for the exchange reactions of hydrogen isotopes between hydrogen and vapor in the water detritiation system, and for the oxidation of tritium on the atmospheric detritiation system. Hydrophobic platinum catalysts are hardly susceptible to water mist and water vapor. Hydrophobic platinum catalysts are produced by supporting platinum directly on hydrophobic polymer beads. For the hydrophobic polymer, styrene - divinyl benzene (SDB) has been applied in Japan. It can be pointed out that the upgrade in catalytic activity of hydrophobic catalyst is expected to downsize the catalytic reactor based on a hard look at a large increase in flow rate in future. The upgrade in catalytic activity of two types of commercial Pt/SDB catalysts was found when they were irradiated with electron beams. After irradiation with electron beams, the catalytic activity was evaluated by means of overall reaction rate constant for the oxidation of tritium. The overall reaction rate constant increased as increase in dose. The constant showed the peak value in the dose between 500 to 1000 kGy. After the peak, the constant decreased as increase in dose. The overall reaction rate constant at the peak was 6 times larger than that evaluated with unirradiated. The mechanical strength of irradiated Pt/SDB kept sound until 1500 kGy. The irradiation is a promising method to the upgrading in catalytic activity of Pt/SDB catalyst.
 system
systemKurosaki, Yuzuru; Takayanagi, Toshiyuki*
Chemical Physics Letters, 406(1-3), p.121 - 125, 2005/04
no abstracts in English
Lis, S.*; Kimura, Takaumi; Yoshida, Zenko; But, S.*
Journal of Alloys and Compounds, 380(1-2), p.173 - 176, 2004/10
Times Cited Count:6 Percentile:41.05(Chemistry, Physical)no abstracts in English
 potential energy surfaces for the lowest three doublet states (1
potential energy surfaces for the lowest three doublet states (1 A', 2
A', 2 A', and 1
A', and 1 A") of the BrH
A") of the BrH system
systemKurosaki, Yuzuru; Takayanagi, Toshiyuki
Journal of Chemical Physics, 119(15), p.7838 - 7856, 2003/10
Times Cited Count:26 Percentile:63.55(Chemistry, Physical)Adiabatic potential energy surfaces of the lowest three doublet states (1 A', 2
A', 2 A', and 1
A', and 1 A") for the BrH
A") for the BrH system have been calculated globally using the MRCI+Q method with the aug-cc-pVTZ basis set. Spin-orbit effects were considered on the basis of Breit-Pauli Hamiltonian. The calculated adiabatic energies were fitted to the analytical functional form of many-body expansion. The barrier heights of the abstraction and exchange reactions on the ground-state PES were calculated to be 1.28 and 11.71 kcal mol
system have been calculated globally using the MRCI+Q method with the aug-cc-pVTZ basis set. Spin-orbit effects were considered on the basis of Breit-Pauli Hamiltonian. The calculated adiabatic energies were fitted to the analytical functional form of many-body expansion. The barrier heights of the abstraction and exchange reactions on the ground-state PES were calculated to be 1.28 and 11.71 kcal mol , respectively, at the MRCI+Q/aug-cc-pVTZ level of theory. The fits for the three PESs were successful within the accuracy of 0.1 kcal mol
, respectively, at the MRCI+Q/aug-cc-pVTZ level of theory. The fits for the three PESs were successful within the accuracy of 0.1 kcal mol . Thermal rate constants for the abstraction and exchange reactions and their isotopic variants were calculated with the fitted 1
. Thermal rate constants for the abstraction and exchange reactions and their isotopic variants were calculated with the fitted 1 A' PES using the ICVT/LAG method. The calculated rate constants for the abstarction reactions agree fairly well with experiment but those for the exchange reactions were much smaller than experiment, which suggests that the reliable experimental data are still insufficient.
A' PES using the ICVT/LAG method. The calculated rate constants for the abstarction reactions agree fairly well with experiment but those for the exchange reactions were much smaller than experiment, which suggests that the reliable experimental data are still insufficient.
Ohashi, Hirofumi; Inagaki, Yoshiyuki
JAERI-Tech 2003-046, 47 Pages, 2003/05
no abstracts in English
Nagase, Fumihisa; Otomo, Takashi; Uetsuka, Hiroshi
Journal of Nuclear Science and Technology, 40(4), p.213 - 219, 2003/04
Times Cited Count:69 Percentile:96.68(Nuclear Science & Technology)Isothermal oxidation tests in flowing steam were performed on low-Sn Zircaloy-4 cladding tubes over the wide temperature range from 773 to 1573 K in order to obtain oxidation kinetics applicable to various loss-of-coolant accident conditions of LWRs. The oxidation generally obeys a parabolic rate law for the examined time range up to 3600s at temperatures from 1273 K to 1573K, and for a limited time range up to 900s from 773 to 1253 K. A cubic rate law is preferable for evaluating the longer-term oxidation at 1253 K and below. The parabolic rate law constant and the cubic rate law constant for measured weight gain were evaluated at every examined temperature, and Arrhenius-type equations were determined in order to describe the temperature dependence of the rate constants. It was indicated that the change of the oxidation kinetics from the cubic to the parabolic rate and the discontinuities in the temperature dependence of the rate constants are caused by the monoclinic/tetragonal phase transformation of ZrO .
.
Hirota, Koichi; Arai, Hidehiko; Hashimoto, Shoji
Bulletin of the Chemical Society of Japan, 73(12), p.2719 - 2724, 2000/12
Times Cited Count:7 Percentile:38.32(Chemistry, Multidisciplinary)no abstracts in English
Saeki, Masakatsu; Nakada, Masami; ; Nakamura, Akio
SIF Conf. Proc., Vol. 50 (ICAME-95), 0, p.119 - 122, 1996/00
no abstracts in English
Noguchi, Hiroshi
Fusion Technology, 27(2T), p.56 - 61, 1995/03
no abstracts in English
; G.R.Sunaryo*; Ishigure, Kenkichi *
Journal of Physical Chemistry, 98(19), p.5164 - 5173, 1994/00
Times Cited Count:78 Percentile:91.6(Chemistry, Physical)no abstracts in English
Murata, Mikio; Noguchi, Hiroshi
Nihon Genshiryoku Gakkai-Shi, 34(2), p.149 - 152, 1992/02
Times Cited Count:2 Percentile:43.73(Nuclear Science & Technology)no abstracts in English
Noguchi, Hiroshi; Murata, Mikio
Nihon Genshiryoku Gakkai-Shi, 33(4), p.360 - 362, 1991/04
Times Cited Count:2 Percentile:51.2(Nuclear Science & Technology)no abstracts in English
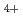 and N
and N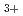 with electrons in atmospheric pressure nitrogen
with electrons in atmospheric pressure nitrogenIkezoe, Yasumasa; *; *
Chemical Physics Letters, 177(4-5), p.366 - 370, 1991/03
Times Cited Count:12 Percentile:45.9(Chemistry, Physical)no abstracts in English
Arakawa, Kazuo;
Shitsuryo Bunseki, 31(4), p.251 - 257, 1983/00
no abstracts in English
Arakawa, Kazuo; Seguchi, Tadao; *
Shitsuryo Bunseki, 29(3), p.257 - 265, 1981/00
no abstracts in English
; ;
JAERI-M 8187, 21 Pages, 1979/03
no abstracts in English
*; Tachikawa, Enzo;
Nuclear Technology, 42(2), p.172 - 179, 1979/00
Times Cited Count:4no abstracts in English