Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ueda, Yuki; Eguchi, Ayano; Tokunaga, Kohei; Kikuchi, Kei*; Sugita, Tsuyoshi; Okamura, Hiroyuki; Naganawa, Hirochika
Industrial & Engineering Chemistry Research, 61(19), p.6640 - 6649, 2022/05
Times Cited Count:1 Percentile:12.67(Engineering, Chemical)no abstracts in English
Atanassova, M.*; Okamura, Hiroyuki; Eguchi, Ayano; Ueda, Yuki; Sugita, Tsuyoshi; Shimojo, Kojiro
Analytical Sciences, 34(8), p.973 - 978, 2018/08
Times Cited Count:17 Percentile:59.57(Chemistry, Analytical)The distribution constants of 4-benzoyl-3-phenyl-5-isoxazolone (HPBI) and deprotonated one (PBI ) between hydrophobic ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C
) between hydrophobic ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C C
C im][Tf
im][Tf N]) and aqueous phases were determined, together with the acid-dissociation constant of HPBI. The solvent extraction of three selected lanthanoid ions (La
N]) and aqueous phases were determined, together with the acid-dissociation constant of HPBI. The solvent extraction of three selected lanthanoid ions (La , Eu
, Eu , and Lu
, and Lu ) with HPBI from aqueous nitrate phase into [C
) with HPBI from aqueous nitrate phase into [C C
C im][Tf
im][Tf N] has been investigated. Application of the ionic liquid as the extracting phase greatly enhanced the extraction performance of HPBI for lanthanoid ions compared with that in the chloroform system. The composition of the extracted species was established to be anionic tetrakis entities, Ln(PBI)
N] has been investigated. Application of the ionic liquid as the extracting phase greatly enhanced the extraction performance of HPBI for lanthanoid ions compared with that in the chloroform system. The composition of the extracted species was established to be anionic tetrakis entities, Ln(PBI)
 , for light, middle, and heavy lanthanoid ions in an ionic environment.
, for light, middle, and heavy lanthanoid ions in an ionic environment.
Shibata, Masahiro; Sawada, Atsushi; Tachi, Yukio; Hayano, Akira; Makino, Hitoshi; Wakasugi, Keiichiro; Mitsui, Seiichiro; Oda, Chie; Kitamura, Akira; Osawa, Hideaki; et al.
JAEA-Research 2013-037, 455 Pages, 2013/12
Following FY2011, JAEA and NUMO have conducted a collaborative research work which is designed to enhance the methodology of repository design and performance assessment in preliminary investigation stage. With regard to (1) study on rock suitability in terms of hydrology, the tree diagram of methodology of groundwater travel time has been extended for crystalline rock, in addition, tree diagram for sedimentary rock newly has been organized. With regard to (2) study on scenario development, the existing approach has been improved in terms of a practical task, and applied and tested for near field focusing on the buffer. In addition, the uncertainty of some important processes and its impact on safety functions are discussed though analysis. With regard to (3) study on setting radionuclide migration parameters, the approaches for parameter setting have been developed for sorption for rocks and solubility, and applied and tested through parameter setting exercises for key radionuclides.
 -oxide
-oxideEguchi, Ayano; Morita, Kotaro*; Okamura, Hiroyuki; Hirayama, Naoki*
no journal, ,
2-Mercaptopyridine  -oxide (HSPyO) is a bidentate ligand with a hard O-donor and a soft S-donor. This ligand is expected to be effective in an ionic liquid (IL) chelate extraction because of its high solubility in ILs. In this study, we investigated the IL chelate extraction behavior of Al(III), Ga(III), and In(III) into ILs, 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides ([C
-oxide (HSPyO) is a bidentate ligand with a hard O-donor and a soft S-donor. This ligand is expected to be effective in an ionic liquid (IL) chelate extraction because of its high solubility in ILs. In this study, we investigated the IL chelate extraction behavior of Al(III), Ga(III), and In(III) into ILs, 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides ([C mim][Tf
mim][Tf N], n = 2, 4, 8), using HSPyO as an extractant. The extraction selectivity order was In(III)
N], n = 2, 4, 8), using HSPyO as an extractant. The extraction selectivity order was In(III)  Ga(III), whereas Al(III) was not extracted. Therefore, HSPyO was favorable for the extraction of soft In(III). The extractability order for solvents was ILs
Ga(III), whereas Al(III) was not extracted. Therefore, HSPyO was favorable for the extraction of soft In(III). The extractability order for solvents was ILs  chloroform in the Ga(III) extraction, indicating that ILs exhibit high extraction performance. In addition, it was implied that the extracted species were the neutral complexes, M(SPyO)
chloroform in the Ga(III) extraction, indicating that ILs exhibit high extraction performance. In addition, it was implied that the extracted species were the neutral complexes, M(SPyO) , for all metals.
, for all metals.
Eguchi, Ayano; Morita, Kotaro*; Okamura, Hiroyuki; Hirayama, Naoki*
no journal, ,
In chelate extraction of metal ions into conventional organic solvents, the metals are generally extracted as uncharged complexes. Moreover, coordinatively saturated (unhydrated) complexes are preferable as extracted species. In ionic liquid (IL) chelate extraction, on the contrary, also charged species or coordinatively unsaturated (hydrated) species can be extracted. In this study, we investigated the IL chelate extraction behavior of trivalent metal ions, Fe(III), Al(III), Ga(III) and In(III), into 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides ([C mim][Tf
mim][Tf N], n = 2, 4, 8) using 8-quinolinol (H
N], n = 2, 4, 8) using 8-quinolinol (H Q
Q ) and 2-mercaptopyridine
) and 2-mercaptopyridine  -oxide (H
-oxide (H SPyO
SPyO ) as bidentate ligands. In the case of H
) as bidentate ligands. In the case of H Q
Q , only uncharged M
, only uncharged M Q
Q
 was extracted into chloroform, whereas coordinatively unsaturated cationic complex [M
was extracted into chloroform, whereas coordinatively unsaturated cationic complex [M Q
Q
 ]
] was also extracted into ILs by ion exchange. In the case of H
was also extracted into ILs by ion exchange. In the case of H SPyO
SPyO , it was implied that uncharged M
, it was implied that uncharged M (SPyO)
(SPyO)
 was extracted into both chloroform and ILs. Therefore, the extracted species depends on the ligands in ILs.
was extracted into both chloroform and ILs. Therefore, the extracted species depends on the ligands in ILs.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the hydrophobicity and structure of an ionic liquid (IL) anion on the extraction of trivalent lanthanoid ions (Ln(III) = La, Nd, Eu, Dy, Lu) using 2-thenoyltrifluoroacetone (Htta) was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different perfluoroalkyl (Rf) chain length (n=0, 1, 2, 4) were synthesized and used as the extraction solvent. The extraction selectivity of Ln(III) was in the order of Lu 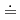 Dy
Dy  Eu
Eu  Nd
Nd  La for all ILs. In addition, there was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. The results implied that the extracted species can be adjusted by Rf chain length of IL anions.
La for all ILs. In addition, there was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. The results implied that the extracted species can be adjusted by Rf chain length of IL anions.
Eguchi, Ayano; Morita, Kotaro*; Okamura, Hiroyuki; Hirayama, Naoki*
no journal, ,
In chelate extraction of metal ions into organic solvents, formation of their uncharged complexes with ligands is required. Moreover, coordinatively saturated (unhydrated) complexes are more preferable as extracted species. On the other hand, in ionic liquid chelate extraction, ionic liquid can extract charged species and coordinatively unsaturated (hydrated) species. In this study, the effects of bidentate ligands on ionic liquid chelate extraction of trivalent metal ions, Fe(III), Al(III), Ga(III) and In(III), into 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imides ([C mim][Tf
mim][Tf N], n=2,4,8) was studied using 8-quinolinol (HQ) and 2-mercaptopyridine
N], n=2,4,8) was studied using 8-quinolinol (HQ) and 2-mercaptopyridine  -oxide (HSPyO) as extractants. The extraction selectivity order for trivalent metal ions was Fe
-oxide (HSPyO) as extractants. The extraction selectivity order for trivalent metal ions was Fe 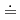 Ga
Ga  In
In  Al for HQ and Fe
Al for HQ and Fe  In
In  Ga
Ga 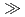 Al for HSPyO. Namely, HSPyO was favorable for extraction of In(III). The extractability of Ga(III) was in the order of [C
Al for HSPyO. Namely, HSPyO was favorable for extraction of In(III). The extractability of Ga(III) was in the order of [C mim][Tf
mim][Tf N]
N]  [C
[C mim][Tf
mim][Tf N]
N]  [C
[C mim][Tf
mim][Tf N]
N]  chloroform for HSPyO, whereas reversed order was obtained for HQ. These results were seemed to be based on difference in concentration of deprotonated bidentate ligands in water.
chloroform for HSPyO, whereas reversed order was obtained for HQ. These results were seemed to be based on difference in concentration of deprotonated bidentate ligands in water.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the perfluoroalkyl (Rf) chain length of the ionic liquid (IL) anion on the extraction of trivalent lanthanoid ions (Ln(III) = La, Nd, Eu, Dy, Lu) using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different Rf chain length (n = 1-4) were synthesized and used as the extraction solvent. There was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. It was revealed that the extracted complex depends on even or odd number of the Rf chain length n.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the perfluoroalkyl (Rf) chain length of the ionic liquid (IL) anion on the IL chelate extraction was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different Rf chain length (n = 1 - 4) were synthesized and used as extraction solvents. Herein, the extraction of trivalent lanthanoids (Ln(III) = La, Nd, Eu, Dy and Lu) with an extractant, 2-thenoyltrifluoroacetone, was studied. It was revealed that the extracted complex depends on even or odd number of the Rf chain length n.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the perfluoroalkyl (Rf) chain length of the ionic liquid (IL) anion on the IL chelate extraction of trivalent lanthanoids using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different Rf chain length (n = 1-4) were synthesized and used as extraction solvents. The extracted species differed depending on odd-even number of the Rf chain length n, and it was found that the Rf chain length of IL anion has the odd-even effect on the extracted species. The fluorescence lifetime of the extracted Eu(III) complex was measured and the number of coordinating water of Eu(III) was determined. As a result, the Eu(III) complex did not have coordinating water. It was suggested that the Rf chain length of the IL anion does not affect the number of coordinating water of the extracted complex.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
Physical and chemical properties of ionic liquids (ILs) can be tuned by changing the combination of an anion and a cation. In the solvent extraction of metal ions, it is well known that the structure and alkyl chain length of the IL cation have a great effect on the extractability. However, the structural effect of the IL anion on the extractability is hardly clarified. In this study, the effect of the side chain length of the IL anion on the IL chelate extraction of trivalent lanthanoids using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different side chain lengths (n = 1-4) were synthesized and used as extraction solvents. When using ILs which side chain lengths n were even (n = 2, 4), the extracted species were different from those when using ILs that n were odd (n = 1, 3) and the IL having hexafluorophosphate anion. It was, therefore, implied that the extracted species can be controlled by the side chain length of the IL anion.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
Physical and chemical properties of ionic liquids (ILs) can be tuned by changing the combination of an anion and a cation composing an IL. In the solvent extraction of metal ions, it is considered that IL anions affect extraction by the interaction IL anions and metal ions. However, the structural effect of the IL anions on extraction is hardly clarified. In this study, the effect of the side chain of the IL anions on the IL chelate extraction of trivalent lanthanoids using 2-thenoyltrifluoroacetone was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different carbon number of side chain n (n = 1-4) were synthesized and used as extraction solvents. When n was even, the extracted species were different from those when n was odd. This result was considered to be due to the different IL solvation effects on the extracted complexes depending on n. It was implied that the side chain in the IL anions was involved in the extraction mechanism by affecting solvation of extracted complexes.
Eguchi, Ayano; Kimuro, Shingo; Amano, Yuki; Tachi, Yukio
no journal, ,
no abstracts in English
Kimuro, Shingo; Iwata, Hajime; Eguchi, Ayano; Nishikawa, Yoshiaki*; Tachi, Yukio
no journal, ,
no abstracts in English
Eguchi, Ayano; Kimuro, Shingo; Amano, Yuki; Tachi, Yukio
no journal, ,
no abstracts in English