Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 at the single-atom level
at the single-atom levelSteinegger, P.*; Asai, Masato; Dressler, R.*; Eichler, R.*; Kaneya, Yusuke*; Mitsukai, Akina*; Nagame, Yuichiro; Piguet, D.*; Sato, Tetsuya; Sch del, M.; et al.
del, M.; et al.
Journal of Physical Chemistry C, 120(13), p.7122 - 7132, 2016/04
Times Cited Count:23 Percentile:60.16(Chemistry, Physical)A new experimental method "vacuum chromatography" has been developed to measure adsorption enthalpy of superheavy elements, and its feasibility has been examined using short-lived thallium isotopes. The short-lived thallium isotopes were produced at the JAEA tandem accelerator. The thallium ion beam prepared with an on-line isotope separator which ionized and mass-separated the thallium isotopes was injected into an isothermal vacuum chromatography apparatus. A temperature-dependent adsorption property of thallium atom on SiO surface were measured. The adsorption enthalpy of thallium was determined to be 158 kJ/mol. The thallium is a homolog of element 113. Thus, the vacuum chromatography developed in this study enables us to perform chemical experiments for short-lived superheavy elements with half-lives of a order of one second.
surface were measured. The adsorption enthalpy of thallium was determined to be 158 kJ/mol. The thallium is a homolog of element 113. Thus, the vacuum chromatography developed in this study enables us to perform chemical experiments for short-lived superheavy elements with half-lives of a order of one second.
Vascon, A.; Wiehl, N.*; Runke, J.*; Drebert, J.*; Reich, T.*; Trautmann, N.*; Cremer, B.*; K gler, T.*; Beyer, R.*; Junghans, A.*; et al.
gler, T.*; Beyer, R.*; Junghans, A.*; et al.
no journal, ,
Toyoshima, Atsushi; Miyashita, Sunao*; Oe, Kazuhiro*; Kitayama, Yuta*; Lerum, H. V.*; Goto, Naoya*; Kaneya, Yusuke; Komori, Yukiko*; Mitsukai, Akina*; Vascon, A.; et al.
no journal, ,
no abstracts in English
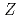 = 102)
= 102)Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke; Tsukada, Kazuaki; Toyoshima, Atsushi; Vascon, A.; Takeda, Shinsaku; Mitsukai, Akina*; Nagame, Yuichiro; Ichikawa, Shinichi; et al.
no journal, ,
In order to determine the IP of the heavy elements, we have developed a novel measurement method based on a surface ionization technique by using a surface ionization ion source coupled to a He/CdI gas-jet transport system for an Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator facility. In this work, we have determined IP value of No by using the method. In a surface ionization process, an ionization efficiency of an atom depends on its IP. To obtain a relationship between IP and ionization efficiency in present system, we measured ionization efficiencies of various short-lived isotopes. Ionization efficiency of
gas-jet transport system for an Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator facility. In this work, we have determined IP value of No by using the method. In a surface ionization process, an ionization efficiency of an atom depends on its IP. To obtain a relationship between IP and ionization efficiency in present system, we measured ionization efficiencies of various short-lived isotopes. Ionization efficiency of 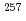 No produced in the
No produced in the  Cm(
Cm( C, 4n) reaction was also measured. Measured ionization efficiency of
C, 4n) reaction was also measured. Measured ionization efficiency of 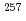 No was 0.8%, which yields IP value of No to be 6.6 eV. This value is in a good agreement with the value which has been evaluated by extrapolation from those of the lighter actinide elements, 6.65 eV.
No was 0.8%, which yields IP value of No to be 6.6 eV. This value is in a good agreement with the value which has been evaluated by extrapolation from those of the lighter actinide elements, 6.65 eV.
Vascon, A.; Wiehl, N.*; Runke, J.*; Drebert, J.*; Reich, T.*; Trautmann, N.*; Cremer, B.*; K gler, T.*; Beyer, R.*; Junghans, A. R.*; et al.
gler, T.*; Beyer, R.*; Junghans, A. R.*; et al.
no journal, ,
Toyoshima, Atsushi; Oe, Kazuhiro*; Asai, Masato; Attallah, M. F.*; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Kaneko, Masashi*; Kaneya, Yusuke; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Due to short half-lives less than 10 s and extremely low production rates, transactinide elements heavier than seaborgium (Sg) are produced on an atom per hour scale. Therefore, a continuous rapid chemistry assembly is required to study aqueous-phase chemistry of these heaviest elements. In the present study, we started developments of a continuous chemistry assembly. Our first attempt was made in on-line experiments with Mo and W, lighter homologs of Sg, to optimize a chemistry assembly consisting of a newly developed membrane degasser as an interface between gas-jet and aqueous phase, a flow electrolytic column apparatus utilized to control oxidation states of Mo and W ions, and the continuous liquid-liquid extraction apparatus of SISAK for separation. In the conference, present status of the developments will be presented.
Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke*; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Takeda, Shinsaku*; Vascon, A.*; Sakama, Minoru*; Sato, Daisuke*; et al.
no journal, ,
The first ionization potential (IP ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
Nagame, Yuichiro; Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke*; Makii, Hiroyuki; Mitsukai, Akina; Osa, Akihiko; Sch del, M.*; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
del, M.*; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
no journal, ,
Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke*; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Takeda, Shinsaku*; Vascon, A.*; Sakama, Minoru*; Sato, Daisuke*; et al.
no journal, ,
The first ionization potential (IP ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.