Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sawaguchi, Takuma; Yamaguchi, Tetsuji; Iida, Yoshihisa; Tanaka, Tadao; Kitagawa, Isamu
Clay Minerals, 48(2), p.411 - 422, 2013/05
Times Cited Count:2 Percentile:6.45(Chemistry, Physical)Diffusive transport of Cs in compacted sand-bentonite mixtures was studied by a through diffusion method. Experiments were performed under variable aqueous compositions. Effective diffusivity (
in compacted sand-bentonite mixtures was studied by a through diffusion method. Experiments were performed under variable aqueous compositions. Effective diffusivity (
 ) values of 5.2E-10
) values of 5.2E-10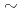 5.9E-9 m
5.9E-9 m s
s were obtained. The variation was somewhat large in the
were obtained. The variation was somewhat large in the 
 values. Apparent diffusivity (
values. Apparent diffusivity (
 ) values, on the other hand, were 2.0E-12
) values, on the other hand, were 2.0E-12  6.2E-12 m
6.2E-12 m s
s , which shows small variation. The results indicate that, in applying Fick's 1st law of diffusion, diffusive flux is proportional to the apparent concentration gradient of Cs in the sand-bentonite mixture rather than the gradient of Cs concentration in pore water. Since the apparent concentration gradient in sand-bentonite mixtures is nearly equal to the gradient of adsorbed Cs, diffusion of Cs under adsorbed state would be the main mechanism of diffusion of Cs in sand-bentonite mixtures.
, which shows small variation. The results indicate that, in applying Fick's 1st law of diffusion, diffusive flux is proportional to the apparent concentration gradient of Cs in the sand-bentonite mixture rather than the gradient of Cs concentration in pore water. Since the apparent concentration gradient in sand-bentonite mixtures is nearly equal to the gradient of adsorbed Cs, diffusion of Cs under adsorbed state would be the main mechanism of diffusion of Cs in sand-bentonite mixtures.
 concrete/clay interactions at the Tournemire URL
concrete/clay interactions at the Tournemire URLYamaguchi, Tetsuji; Kataoka, Masaharu; Sawaguchi, Takuma; Mukai, Masayuki; Hoshino, Seiichi; Tanaka, Tadao; Marsal, F.*; Pellegrini, D.*
Clay Minerals, 48(2), p.185 - 197, 2013/05
Times Cited Count:3 Percentile:9.44(Chemistry, Physical)Highly alkaline environments induced by cement based materials are likely to deteriorate the physical and/or chemical properties of the bentonite buffer materials in radioactive waste repositories. Predicting long-term alteration of concrete/clay systems requires physico-chemical models and a number of input parameters. In order to provide reliability to the long-term prediction of bentonite buffer performance under disposal conditions, it is necessary to develop and verify reactive transport codes for concrete/clay systems. In this study, a PHREEQC-based, reactive transport analysis code (MC-CEMENT ver.2) was developed and was verified by comparing results of the calculations with  observations of the mineralogical evolution at the concrete/argillite interface. The calculation reproduced the observations such as the mineralogical changes limited within one cm in thickness, formation of CaCO
observations of the mineralogical evolution at the concrete/argillite interface. The calculation reproduced the observations such as the mineralogical changes limited within one cm in thickness, formation of CaCO and CSH, dissolution of quartz, decrease of porosity in argillite and increase in concrete. These agreements indicate possibility that the models based on lab-scale (
and CSH, dissolution of quartz, decrease of porosity in argillite and increase in concrete. These agreements indicate possibility that the models based on lab-scale ( 1 y) experiments can be applied to longer time scale. The fact that the calculation did not reproduce the dissolution of clays and the formation of gypsum indicates that there is still room for improvement in our model.
1 y) experiments can be applied to longer time scale. The fact that the calculation did not reproduce the dissolution of clays and the formation of gypsum indicates that there is still room for improvement in our model.
Yamaguchi, Tetsuji; Sawaguchi, Takuma; Tsukada, Manabu; Kadowaki, Mitsushi*; Tanaka, Tadao
Clay Minerals, 48(2), p.403 - 410, 2013/05
Times Cited Count:9 Percentile:26(Chemistry, Physical)Montmorillonite is the main constituent of bentonite clay buffer materials in radioactive waste repositories. Highly alkaline environments induced by cement based materials are likely to alter montmorillonite, and to deteriorate the physical and/or chemical properties of the buffer materials. The deterioration may cause variation in hydraulic conductivity of the buffer and induce major uncertainties in the radionuclide migration analysis. Empirical data on the variation of hydraulic conductivity are, however, scarce mainly because the alteration of compacted buffer materials, sand-bentonite mixture specimen, is extremely slow. In this study, laboratory experiments were performed to observe changes in hydraulic conductivity of sand-bentonite mixtures accompanied with their alkaline alteration using NaOH based solutions at 80-90  C. Three types of experiments proved that the alkaline alteration of bentonite buffer can increase the hydraulic conductivity. The data obtained in this study are useful for verification of the code that will be used for assessing the alteration.
C. Three types of experiments proved that the alkaline alteration of bentonite buffer can increase the hydraulic conductivity. The data obtained in this study are useful for verification of the code that will be used for assessing the alteration.
 in compacted sodium montmorillonite as a function of porewater salinity
in compacted sodium montmorillonite as a function of porewater salinityTachi, Yukio; Yotsuji, Kenji
Proceedings of 5th International Meeting on Clays in Natural and Engineered Barriers for Radioactive Waste Confinement (USB Flash Drive), p.899 - 900, 2012/10
Sorption and diffusion of radionuclides in compacted bentonite are key processes in the safe geological disposal. The effects of porewater salinity on Sr diffusion and sorption in compacted montmorillonite were investigated by experimental and modeling approaches. Effective diffusion coefficients (De) and distribution coefficients (Kd) of Sr in montmorillonite compacted to dry density of 800 kg/m were measured under four different salinities (0.01-0.5 M NaCl). The De decreased drastically with increasing porewater salinity. The Kd values decreased with increasing salinity, which are consistent with Kd values obtained by batch sorption. These results were interpreted by the ISD model coupling the thermodynamic sorption model and the EDL diffusion model in narrow pores. The ISD model can be basically applicable for divalent cations, however is needed to modify for better agreement at low salinity conditions by considering additional factors.
were measured under four different salinities (0.01-0.5 M NaCl). The De decreased drastically with increasing porewater salinity. The Kd values decreased with increasing salinity, which are consistent with Kd values obtained by batch sorption. These results were interpreted by the ISD model coupling the thermodynamic sorption model and the EDL diffusion model in narrow pores. The ISD model can be basically applicable for divalent cations, however is needed to modify for better agreement at low salinity conditions by considering additional factors.
Yotsuji, Kenji; Tachi, Yukio; Nishimaki, Yuichiro*
Proceedings of 5th International Meeting on Clays in Natural and Engineered Barriers for Radioactive Waste Confinement (USB Flash Drive), p.427 - 428, 2012/10
We have developed integrated sorption and diffusion model (ISD model) for assessment of diffusion parameters consistent with sorption processes in compacted bentonite. Conventional ISD model is unsatisfactory because for multivalent cation/anion and complex ion model predictions disagree with experimental data, and because the model contains additional non-physical fitting parameter. Accordingly we extract the factors influencing the assessment from fundamental assumptions of the conventional ISD model. In this study we incorporated the excluded volume effect and the dielectric saturation effect into ISD model. As results of numerical analysis of these models the considering factors influence hardly the effective diffusivity. Therefore it does not mean that the disagreement with experimental data are caused by considering factors in this report.
Wilson, J.*; Watson, C.*; Benbow, S.*; Savage, D.*; Sasamoto, Hiroshi
no journal, ,
Iron steel overpack will be surrounded by bentonite buffer as a engineering barrier system for the high-level radioactive waste disposal. It is concerned that iron-bentonite interactions result in the alteration of montmorillonite to non-swelling Fe-rich minerals. In the present study, reactive transport modeling of iron-bentonite interface evolution has been conducted to evaluate the long-term behavior of bentonite stability. A number of model cases were produced in order to assess which processes are likely to dominate at iron-bentonite interfaces.
Nakabayashi, Ryo*; Elakneswaran, Y.*; Sato, Tsutomu*; Oda, Chie; Yoneda, Tetsuro*; Kaneko, Katsuhiko*
no journal, ,
The objective of this study is to develop a microstructural analysis method by X-ray CT to track the alteration process arising from bentonite and hyperalkaline fluid interactions as a function of time and to use these data to underpin a geochemical model of the alteration process. Advective alteration experiment of bentonite was performed at 80  C (pH = 13.5 at 25
C (pH = 13.5 at 25  C) for 180 days and in-situ analysis by X-ray CT during the experiment was conducted. The geochemical reactive transport code PHREEQC was used to simulate the results of the experiments. Consequently, the formation of secondary minerals (analcime) in the bentonite could be confirmed by X-ray CT at different times and quantified by X-ray CT images analysis. The predicted time-dependency of volume of analcime by a geochemical reactive transport model became consistent with the results obtained by X-ray CT analysis, and the concentration of dissolved Si and Al in the output solution could be replicated.
C) for 180 days and in-situ analysis by X-ray CT during the experiment was conducted. The geochemical reactive transport code PHREEQC was used to simulate the results of the experiments. Consequently, the formation of secondary minerals (analcime) in the bentonite could be confirmed by X-ray CT at different times and quantified by X-ray CT images analysis. The predicted time-dependency of volume of analcime by a geochemical reactive transport model became consistent with the results obtained by X-ray CT analysis, and the concentration of dissolved Si and Al in the output solution could be replicated.