Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Micheau, C.; Ueda, Yuki; Motokawa, Ryuhei; Akutsu, Kazuhiro*; Yamada, Norifumi*; Yamada, Masako*; Moussaoui, S. A.*; Makombe, E.*; Meyer, D.*; Berthon, L.*; et al.
Journal of Molecular Liquids, 401, p.124372_1 - 124372_12, 2024/05
Supramolecular organization of extractant molecules impacts metal ions separation behavior. Probing bulk and interfacial structures of the relevant systems is expected to provide key insights into the metal ion selectivity and kinetic aspects. The supramolecular features of two solvent extraction systems based on malonamide extractants THMA in toluene and DBMA in n-heptane were studied using small-angle X-ray scattering for the organic bulk phases, as well as interfacial tension and neutron reflectivity measurements for the interfaces. In the bulk solution, THMA forms dimeric/trimeric associates but no aggregates in toluene, while DBMA forms large aggregates in n-heptane. On the other hand, THMA accumulates in a diffuse layer at the interface at high THMA concentration, whereas DBMA forms a compact but thinner layer. After Pd(II) extraction, the thickness of interfacial layers decreases in the case of THMA, and totally vanishes in the case of DBMA. Based on these new structural information, two mechanisms are proposed for Pd(II) and Nd(III) extraction with malonamides. In toluene, THMA associates slightly accumulate in the vicinity of the interface, then coordinate Pd(II) and diffuse into the organic bulk phase. In n-heptane, DBMA aggregates adsorb at the interface then pick up Nd(III) cations in their polar cores and finally diffuse into the bulk.
Kumada, Takayuki; Motokawa, Ryuhei; Oba, Yojiro; Nakagawa, Hiroshi; Sekine, Yurina; Micheau, C.; Ueda, Yuki; Sugita, Tsuyoshi; Birumachi, Atsushi; Sasaki, Miki; et al.
Journal of Applied Crystallography, 56(6), p.1776 - 1783, 2023/12
Times Cited Count:1 Percentile:65.66(Chemistry, Multidisciplinary)The combination of the existing position-sensitive photomultiplier and the 3He main detector with focusing devices, and the newly installed front detectors in SANS-J at JRR-3 covers small-angle neutron scattering signals in the range of the magnitude of the scattering vector Q from 0.002 to 6 nm-1 gaplessly with three standard device layouts. The installation of the front detector and a graphical user interface system largely improved the usability of SANS-J.
Micheau, C.; Ueda, Yuki; Motokawa, Ryuhei; Bauduin, P.*; Girard, L.*; Diat, O.*
Langmuir, 39(31), p.10965 - 10977, 2023/07
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)Massey, D.*; Williams, C. D.*; Mu, J.*; Masters, A. J.*; Motokawa, Ryuhei; Aoyagi, Noboru; Ueda, Yuki; Antonio, M. R.*
Journal of Physical Chemistry B, 127(9), p.2052 - 2065, 2023/03
Times Cited Count:0 Percentile:0(Chemistry, Physical)Micheau, C.; Ueda, Yuki; Akutsu, Kazuhiro*; Bourgeois, D.*; Motokawa, Ryuhei
Solvent Extraction and Ion Exchange, 41(2), p.221 - 240, 2023/02
Times Cited Count:1 Percentile:51.1(Chemistry, Multidisciplinary) microphase separation
microphase separationIsozaki, Yuka*; Higashiharaguchi, Seiya*; Kaneko, Naoya*; Yamazaki, Shun*; Taniguchi, Tatsuo*; Karatsu, Takashi*; Ueda, Yuki; Motokawa, Ryuhei
Chemistry Letters, 51(6), p.625 - 628, 2022/06
Times Cited Count:2 Percentile:29.84(Chemistry, Multidisciplinary)Ueda, Yuki; Eguchi, Ayano; Tokunaga, Kohei; Kikuchi, Kei*; Sugita, Tsuyoshi; Okamura, Hiroyuki; Naganawa, Hirochika
Industrial & Engineering Chemistry Research, 61(19), p.6640 - 6649, 2022/05
Times Cited Count:1 Percentile:12.67(Engineering, Chemical)no abstracts in English
Akutsu-Suyama, Kazuhiro*; Yamada, Norifumi*; Ueda, Yuki; Motokawa, Ryuhei; Narita, Hirokazu*
Applied Sciences (Internet), 12(3), p.1215_1 - 1215_10, 2022/02
Times Cited Count:1 Percentile:28.33(Chemistry, Multidisciplinary)no abstracts in English
 ]
] from hydrochloric acid using a simple urea extractant
from hydrochloric acid using a simple urea extractantUeda, Yuki; Morisada, Shintaro*; Kawakita, Hidetaka*; Wenzel, M.*; Weigand, J. J.*; Oto, Keisuke*
Separation and Purification Technology, 277, p.119456_1 - 119456_8, 2021/12
Times Cited Count:5 Percentile:30.31(Engineering, Chemical)no abstracts in English
Ueda, Yuki; Morisada, Shintaro*; Kawakita, Hidetaka*; Oto, Keisuke*
Separations (Internet), 8(9), p.139_1 - 139_15, 2021/09
Times Cited Count:9 Percentile:63.91(Chemistry, Analytical)no abstracts in English
 He spin filter at a pulsed neutron source
He spin filter at a pulsed neutron sourceOkudaira, Takuya; Ueda, Yuki; Hiroi, Kosuke; Motokawa, Ryuhei; Inamura, Yasuhiro; Takata, Shinichi; Oku, Takayuki; Suzuki, Junichi*; Takahashi, Shingo*; Endo, Hitoshi*; et al.
Journal of Applied Crystallography, 54(2), p.548 - 556, 2021/04
Times Cited Count:3 Percentile:33.1(Chemistry, Multidisciplinary)Neutron polarization analysis (NPA) for small-angle neutron scattering (SANS) experiments using a pulsed neutron source was successfully achieved by applying a  He spin filter as a spin analyzer for the scattered neutrons. The
He spin filter as a spin analyzer for the scattered neutrons. The  He spin filter covers a sufficient solid angle for performing SANS experiments, and the relaxation time of the
He spin filter covers a sufficient solid angle for performing SANS experiments, and the relaxation time of the  He polarization is sufficient for continuous use over a few days, thus reaching the typical duration required for a complete set of SANS experiments. Although accurate evaluation of the incoherent neutron scattering, which is predominantly attributable to hydrogen atoms in samples, is practically difficult using calculations based on the sample elemental composition, the developed NPA approach with consideration of the influence of multiple neutron scattering enabled reliable decomposition of the SANS intensity distribution into the coherent and incoherent scattering components. To date, NPA has not been well established as a standard technique for SANS experiments at pulsed neutron sources. This work is anticipated to greatly contribute to the accurate determination of the coherent neutron scattering component for scatterers in various types of organic sample systems in SANS experiments at J-PARC.
He polarization is sufficient for continuous use over a few days, thus reaching the typical duration required for a complete set of SANS experiments. Although accurate evaluation of the incoherent neutron scattering, which is predominantly attributable to hydrogen atoms in samples, is practically difficult using calculations based on the sample elemental composition, the developed NPA approach with consideration of the influence of multiple neutron scattering enabled reliable decomposition of the SANS intensity distribution into the coherent and incoherent scattering components. To date, NPA has not been well established as a standard technique for SANS experiments at pulsed neutron sources. This work is anticipated to greatly contribute to the accurate determination of the coherent neutron scattering component for scatterers in various types of organic sample systems in SANS experiments at J-PARC.
 from nitrate media; Comparison with a conventional organic phosphate
from nitrate media; Comparison with a conventional organic phosphateUeda, Yuki; Kikuchi, Kei*; Tokunaga, Kohei; Sugita, Tsuyoshi; Aoyagi, Noboru; Tanaka, Kazuya; Okamura, Hiroyuki
Solvent Extraction and Ion Exchange, 39(5-6), p.491 - 511, 2021/00
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)no abstracts in English
 solution; Comparison with tri-
solution; Comparison with tri- -butyl phosphate
-butyl phosphateUeda, Yuki; Kikuchi, Kei; Sugita, Tsuyoshi; Motokawa, Ryuhei
Solvent Extraction and Ion Exchange, 37(5), p.347 - 359, 2019/07
Times Cited Count:8 Percentile:34.18(Chemistry, Multidisciplinary)We have newly-designed fluorous phosphate(TFP) for the effective Zr(IV) ion extractant as an alternative extractant against the conventional organic phosphate, tri- -butyl phosphate(TBP). Zr(IV) ion extraction system using the TBP has many problems such as the formation of the third phase during liquid-liquid extraction. Here, we develop the fluorous extraction system based on the TFP-perfluorohexane for the Zr(IV) ion extraction to improve the Zr(IV) ion extraction system with an effective extractability and without the third phase formation. Our main findings were that the significant high extraction performance of the TFP for Zr(IV) ion as compared with TBP, and the origins of the high extraction performance of the TFP are related to the water and HNO
-butyl phosphate(TBP). Zr(IV) ion extraction system using the TBP has many problems such as the formation of the third phase during liquid-liquid extraction. Here, we develop the fluorous extraction system based on the TFP-perfluorohexane for the Zr(IV) ion extraction to improve the Zr(IV) ion extraction system with an effective extractability and without the third phase formation. Our main findings were that the significant high extraction performance of the TFP for Zr(IV) ion as compared with TBP, and the origins of the high extraction performance of the TFP are related to the water and HNO contents in the fluorous phase and the stability of the complex, Zr(No
contents in the fluorous phase and the stability of the complex, Zr(No )
) (TFP)
(TFP) .
.
Atanassova, M.*; Okamura, Hiroyuki; Eguchi, Ayano; Ueda, Yuki; Sugita, Tsuyoshi; Shimojo, Kojiro
Analytical Sciences, 34(8), p.973 - 978, 2018/08
Times Cited Count:17 Percentile:59.57(Chemistry, Analytical)The distribution constants of 4-benzoyl-3-phenyl-5-isoxazolone (HPBI) and deprotonated one (PBI ) between hydrophobic ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C
) between hydrophobic ionic liquid 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C C
C im][Tf
im][Tf N]) and aqueous phases were determined, together with the acid-dissociation constant of HPBI. The solvent extraction of three selected lanthanoid ions (La
N]) and aqueous phases were determined, together with the acid-dissociation constant of HPBI. The solvent extraction of three selected lanthanoid ions (La , Eu
, Eu , and Lu
, and Lu ) with HPBI from aqueous nitrate phase into [C
) with HPBI from aqueous nitrate phase into [C C
C im][Tf
im][Tf N] has been investigated. Application of the ionic liquid as the extracting phase greatly enhanced the extraction performance of HPBI for lanthanoid ions compared with that in the chloroform system. The composition of the extracted species was established to be anionic tetrakis entities, Ln(PBI)
N] has been investigated. Application of the ionic liquid as the extracting phase greatly enhanced the extraction performance of HPBI for lanthanoid ions compared with that in the chloroform system. The composition of the extracted species was established to be anionic tetrakis entities, Ln(PBI)
 , for light, middle, and heavy lanthanoid ions in an ionic environment.
, for light, middle, and heavy lanthanoid ions in an ionic environment.
Ueda, Yuki; Sugita, Tsuyoshi; Okamura, Hiroyuki; Shimojo, Kojiro; Naganawa, Hirochika; Morisada, Shintaro*; Kawakita, Hidetaka*; Oto, Keisuke*
no journal, ,
Platinum group metals (PGMs) are indispensable metals in modern industries and are used for example as active components in automobile gas catalysts, jewellery goods, electronic devices and dental materials. In order to meet the growing demand, high efficient receptors for liquid-liquid extraction of the respective anionic chloro-complexes are required in order to enhance capable winning from either pour geological deposits or complex secondary sources within the Urban Mining. The ligand containing urea or amide groups can form the hydrogen bond with anionic species. The novel urea or amide containing extractants were synthesized and its extraction behavior and mechanism were investigated in this work.
Shimojo, Kojiro; Fujiwara, Iori*; Sugita, Tsuyoshi; Ueda, Yuki; Okamura, Hiroyuki; Oshima, Tatsuya*; Baba, Yoshinari*; Naganawa, Hirochika
no journal, ,
We have synthesized a thiodiglycolamic acid type ligand with a soft donor atom as a novel extractant. Extraction behavior of 56 metal ions using this extractant was comprehensively investigated. By comparing the extraction properties of this extractant with those of a diglycolamic acid type ligand with a hard donor atom, the effect of soft-hard property in the ether-site on extraction characteristics was clarified.
Sugita, Tsuyoshi; Okamura, Hiroyuki; Ueda, Yuki; Naganawa, Hirochika; Shimojo, Kojiro
no journal, ,
Ionic liquid extraction system has unique extraction property. In this study, novel organophosphate type neutral extractant with tridentate structure was synthesized and it's extraction ability and extraction mechanisms were revealed in ionic liquid system.
Ueda, Yuki; Sugita, Tsuyoshi; Okamura, Hiroyuki; Shimojo, Kojiro; Naganawa, Hirochika; Morisada, Shintaro*; Kawakita, Hidetaka*; Oto, Keisuke*
no journal, ,
Platinum group metals (PGMs) are indispensable metals in modern industries and are used for example as active components in automobile gas catalysts, jewelry goods, electronic devices and dental materials. In order to meet the growing demand, high efficient extraction reagents for liquid-liquid extraction of the respective anionic chloro-complexes are required in order to enhance capable winning from either pour geological deposits or complex secondary sources within the Urban Mining. The ligand containing urea or amide groups can form the hydrogen bond with anionic species. The novel urea or amide containing extraction reagents were synthesized and its extraction behavior and mechanism were investigated in this work.
Eguchi, Ayano; Okamura, Hiroyuki; Sugita, Tsuyoshi; Ueda, Yuki; Morita, Kotaro*; Shimojo, Kojiro; Naganawa, Hirochika; Hirayama, Naoki*
no journal, ,
In this study, the effect of the hydrophobicity and structure of an ionic liquid (IL) anion on the extraction of trivalent lanthanoid ions (Ln(III) = La, Nd, Eu, Dy, Lu) using 2-thenoyltrifluoroacetone (Htta) was investigated. Four 1-butyl-3-methylimidazolium bis(perfluoroalkanesulfonyl)imides with different perfluoroalkyl (Rf) chain length (n=0, 1, 2, 4) were synthesized and used as the extraction solvent. The extraction selectivity of Ln(III) was in the order of Lu 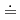 Dy
Dy  Eu
Eu  Nd
Nd  La for all ILs. In addition, there was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. The results implied that the extracted species can be adjusted by Rf chain length of IL anions.
La for all ILs. In addition, there was no significant difference in the extractability among ILs. The extracted species of each Ln(III) in these IL systems were investigated based on the slope of the plots of the logarithmic distribution ratio of Ln(III) as a function of aqueous phase pH. The results implied that the extracted species can be adjusted by Rf chain length of IL anions.
Ueda, Yuki; Morisada, Shintaro*; Kawakita, Hidetaka*; Omi, Kentaro*; Fujita, Mitsuharu*; Weigand, J.*; Shimojo, Kojiro; Naganawa, Hirochika; Oto, Keisuke*
no journal, ,
Platinum group metals (PGMs) are indispensable metals in modern industries and are used for example as active components in automobile gas catalysts, jewelry goods, electronic devices and dental materials. In order to meet the growing demand, high efficient extraction reagents for liquid-liquid extraction of the respective anionic chloro-complexes are required in order to enhance capable winning from either pour geological deposits or complex secondary sources within the Urban Mining. The ligand containing urea or amide groups can form the hydrogen bond with anionic species. The novel urea or amide incorporating extraction reagents were synthesized and its extraction behavior and mechanism were investigated in this work.