再処理高レベル廃液中の放射性核種の群分離に関する研究 DBBP,TBP,D2EMPAおよびD/M2HPA(混合溶媒)による超ウラン元素およびFPの抽出試験
Studies on the partitioning of high level liquid waste; Extraction of transuranium and fission products with DBBP, TBP, D2EHPA and D/M2EHPA (mixed solvent)
岸本 洋一郎; 河田 東海夫*; 根本 慎一*
Kishimoto, Yoichiro; Kawata, Tomio*; not registered
本報告書は,動燃東海事業所技術部廃棄物処理開発室における高レベル廃液の群分離技術開発の成果をまとめたものである。Am及び希士類元素の分離,回収を目的としてDBBP,TBP,D2EHPAおよびD2EHPA/M2EHPA混合溶媒(moe比D/M=1/1)を用いた溶媒抽出法により,バッチ抽出試験およびミキサセトラによる連続抽出試験を行った。 また関連して,活性炭を触媒とした硝酸溶液中におけるPu(4)およびFe(3)のヒドラジンによる還元,乳酸-硝酸混合溶液によるPu(4)/U(6)の分離試験など実施した。 Am(3),Eu(3)およびCe(3)について,DBBP,TBP,E2EHPA,D2EHPA/M2EHPA-HNO3-M-(NO3)-系における抽出データを求めた結果log(E/ 〔ORG〕n)対log〔NO3〕mag,logE対log〔H+〕nagとの間に定量的な直線関係が成立つことを見い出した。これらの元素の見かけの抽出定数(KM)は,DBBP系で1.10(Am)1.26(Eu),1.22(Ce),TBP系で0.0269(Am),0.0324(Eu),0.0219(Ce)D2EHPA系で0.845(Am)更にD2EHPA/M2EHPA混合溶媒系では22(Am),22.7 (Eu)および18.6(Ce)であった。 一方,ヒドラジン-HNO3-活性炭触媒系におけるPu(4),Fe(3)の還元反応では,反応比対反応時間が良い直線性を示し,見かけ上一次反応となった。また,反応速度は活性炭の含まれていない系に比べ300倍増大していた。 乳酸-硝酸混合溶液によるPu(4)/U(6)の分離試験においては,HNO3-U-Pu-TBP系に乳酸が加わると,特にPu(4)の抽出性が低下し,例えば0.5HHNO3-0.3M乳酸の混合溶液ではPu(3)の抽出係数に接近することが判った。
This paper is to present the technology developed in WTL, PNC for the partitioning of actinides in High Level Liquid Waste (HLLW) from nuclear fuel reprocessing plant. Partitioning experiments were primarily made with the batch extraction method, and the counter current continuous extraction method was also examined by using a laboratory scale mixer-settler. Extractants used were Dibutylbutyl Phosphonate (DBBP), Tri-n-butyl Phosphate (TBP), Di (2-ethylhexyl) Phosphoric Acid (D2EHPA) and Di-Mono (2-ethylhexyl) Phosporic Acid mixture (D/M2EHPA, mol ratio D/M=1/1). Other studies were made of the reduction of Pu (IV) or Fe (III) by hydrazine in the nitric acid solution with the active charcoal catalyst. Further studies were also made of the effect of the lactic acid addition on the extraction of Pu (IV) and U (VI) by TBP. The results from the batch extraction of Am (III), Eu(III), and Ce(III) in the DBBP, TBP, D2EHPA or D/M2EHPA-HNO -M
-M (NO
(NO )
) systems showed the linear relationship between log (E/ORG
systems showed the linear relationship between log (E/ORG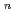 ) vs. log (NO
) vs. log (NO ) aq and log E vs. log (H
) aq and log E vs. log (H )aq. The apparent equilibrium constants (Km) of 1.10 (Am-DBBP), 1.26 (Eu-DBBP), 1.22(Ce-DBBP), 0.0352(Am-TBP), 0.0549(Eu-TBP), 0.0312(Ce-TBP), 0.845(Am-D2EHPA), 22(Am-D/M2EHPA), 22.7(Eu-D/M2EHPA) and 18.7(Ce-D/M2EHPA) were obtained from there relationships. For the reduction of Pu (IV) and Fe (III) in the HNO
)aq. The apparent equilibrium constants (Km) of 1.10 (Am-DBBP), 1.26 (Eu-DBBP), 1.22(Ce-DBBP), 0.0352(Am-TBP), 0.0549(Eu-TBP), 0.0312(Ce-TBP), 0.845(Am-D2EHPA), 22(Am-D/M2EHPA), 22.7(Eu-D/M2EHPA) and 18.7(Ce-D/M2EHPA) were obtained from there relationships. For the reduction of Pu (IV) and Fe (III) in the HNO -hydrazine-active chacoal catalyst system, a good linearity between the reaction ratio and the reduction time was observed, and the reaction was apparently of the first order. The reduction rates of Pu (IV) and Fe (III) in this system were significantly increased by 300 times that observed in the system without the active chacoal catalyst. The addition of lactic acid to the system of HNO
-hydrazine-active chacoal catalyst system, a good linearity between the reaction ratio and the reduction time was observed, and the reaction was apparently of the first order. The reduction rates of Pu (IV) and Fe (III) in this system were significantly increased by 300 times that observed in the system without the active chacoal catalyst. The addition of lactic acid to the system of HNO -U-Pu-TBP lowered the extractability of Pu (IV) to TBP, and the distribution coefficient of Pu (IV), for example, was in the vicinity of that for Pu (III) in the 0.5N HNO
-U-Pu-TBP lowered the extractability of Pu (IV) to TBP, and the distribution coefficient of Pu (IV), for example, was in the vicinity of that for Pu (III) in the 0.5N HNO -0.3M lactic acid soluti
-0.3M lactic acid soluti