Characterization of a monofunctional catalase KatA from radioresistant bacterium 
Kobayashi, Issei*; Tamura, Takashi*; Sghaier, H.; Narumi, Issei; Yamaguchi, Shotaro*; Umeda, Koichi*; Inagaki, Kenji*
Catalase plays a key role in protecting cells against toxic reactive oxygen species. Here we report on the cloning, purification and characterization of a catalase (KatA) from the extremely radioresistant bacterium  . The size of purified
. The size of purified 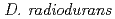 KatA monomer was 65 kDa while gel filtration revealed that the size of the enzyme was 240 kDa, suggesting that KatA formed a homotetramer in solution. Purified KatA displayed a final specific activity of 68,800 U/mg of protein. The catalase activity of KatA was inhibited by sodium azide, sodium cyanide and 3-amino-1, 2, 4-triazole. The absorption spectrum of KatA exhibited a Soret band at 408 nm. The position of the spectral peak remained unchanged following reduction of KatA with dithionite. No peroxidase activity was found for KatA. These results demonstrate that
KatA monomer was 65 kDa while gel filtration revealed that the size of the enzyme was 240 kDa, suggesting that KatA formed a homotetramer in solution. Purified KatA displayed a final specific activity of 68,800 U/mg of protein. The catalase activity of KatA was inhibited by sodium azide, sodium cyanide and 3-amino-1, 2, 4-triazole. The absorption spectrum of KatA exhibited a Soret band at 408 nm. The position of the spectral peak remained unchanged following reduction of KatA with dithionite. No peroxidase activity was found for KatA. These results demonstrate that 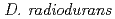 KatA is a typical monofunctional heme-containing catalase. The stability of KatA with respect to H
KatA is a typical monofunctional heme-containing catalase. The stability of KatA with respect to H O
O stress was superior to that of commercially available
stress was superior to that of commercially available 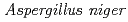 and bovine liver catalases. The relative abundance of KatA in cells in addition to the H
and bovine liver catalases. The relative abundance of KatA in cells in addition to the H O
O resistance property may play a role in the survival strategy of
resistance property may play a role in the survival strategy of 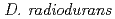 against oxidative damage.
against oxidative damage.