Evaluation of  Cu-labeled DOTA-
Cu-labeled DOTA-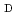 -Phe
-Phe -Tyr
-Tyr -octreotide (
-octreotide ( Cu-DOTA-TOC) for imaging somatostatin receptor-expressing tumors
Cu-DOTA-TOC) for imaging somatostatin receptor-expressing tumors
Hanaoka, Hirofumi*; Tominaga, Hideyuki*; Yamada, Keiichi*; Paudyal, P.*; Iida, Yasuhiko*; Watanabe, Shigeki; Paudyal, B.*; Higuchi, Tetsuya*; Oriuchi, Noboru*; Endo, Keigo*
In-111 (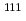 In)-labeled octreotide has been clinically used for imaging somatostatin receptor-positive tumors, and radiolabeled octreotide analogs for positron emission tomography (PET) have been developed. The aim of this study is to produce and fundamentally examine a
In)-labeled octreotide has been clinically used for imaging somatostatin receptor-positive tumors, and radiolabeled octreotide analogs for positron emission tomography (PET) have been developed. The aim of this study is to produce and fundamentally examine a  Cu-labeled octreotide analog,
Cu-labeled octreotide analog,  Cu-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-
Cu-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-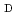 -Phe
-Phe -Tyr
-Tyr -octreotide (
-octreotide ( Cu-DOTA-TOC).
Cu-DOTA-TOC).  Cu-DOTA-TOC can be produced in amounts efficient for clinical study with high radiochemical yield.
Cu-DOTA-TOC can be produced in amounts efficient for clinical study with high radiochemical yield.  Cu-DOTA-TOC was stable in vitro, but time-dependent transchelation to protein was observed after injection into mice. In biodistribution studies, the radioactivity of
Cu-DOTA-TOC was stable in vitro, but time-dependent transchelation to protein was observed after injection into mice. In biodistribution studies, the radioactivity of  Cu was higher than that of
Cu was higher than that of 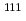 In in all organs except kidney. In tumor-bearing mice,
In in all organs except kidney. In tumor-bearing mice,  Cu-DOTA-TOC showed a high accumulation in the tumors, and the tumor-to-blood ratio reached as high as 8.81
Cu-DOTA-TOC showed a high accumulation in the tumors, and the tumor-to-blood ratio reached as high as 8.81  1.17 at 6 h after administration.
1.17 at 6 h after administration.  Cu-DOTA-TOC showed significantly higher accumulation in the tumor than
Cu-DOTA-TOC showed significantly higher accumulation in the tumor than  Cu-TETA-OC and
Cu-TETA-OC and  Cu-DOTA-OC. PET showed a very clear image of the tumor, which was comparable to that of
Cu-DOTA-OC. PET showed a very clear image of the tumor, which was comparable to that of  F-FDG PET and very similar to that of
F-FDG PET and very similar to that of  Cu-TETA-OC.
Cu-TETA-OC.  Cu-DOTA-TOC clearly imaged a somatostatin receptor-positive tumor and seemed to be a potential PET tracer in the clinical phase.
Cu-DOTA-TOC clearly imaged a somatostatin receptor-positive tumor and seemed to be a potential PET tracer in the clinical phase.