Effect of impurity elements in metal on the corrosion behavior of carbon steel in carbonate aqueous solution and synthetic seawater
Taniguchi, Naoki  ; Suzuki, Hiroyuki*; Naito, Morimasa
; Suzuki, Hiroyuki*; Naito, Morimasa 
Corrosion of metal is an interaction between the material and the environment, so that the corrosion behavior of carbon steel overpack might be affected by not only the environmental factors but also the material factors. In this study, the effect of general impurities in carbon steel such as C, Si, Mn, P and S on the electrochemical behavior and the corrosion rate were studied using carbonate aqueous solution and synthetic seawater. The experimental results were summarized as follows; (1) The effect of the impurities on the critical passivation current density, 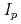 and the passive current density,
and the passive current density, 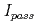 were small in 0.01M carbonate aqueous solution at pH10. (2) Breakdown of passive film and increase in anodic dissolution were observed in the tests for high Si condition of 0.73% and 0.97%. (3) In buffer material saturated with 0.01M carbonate aqueous solution, no passivation was observed and the effect of impurities on the anodic polarization behavior was small. (4) The corrosion rate of carbon steel in seawater was increased with the concentration of impurities. Among the impurity elements, the effects of P and Mn were relatively large. (5) It was inferred that the increase in corrosion rates in synthetic seawater by the addition of Si, Mn and P was promoted by the activation of hydrogen evolution reaction as a cathodic reaction.
were small in 0.01M carbonate aqueous solution at pH10. (2) Breakdown of passive film and increase in anodic dissolution were observed in the tests for high Si condition of 0.73% and 0.97%. (3) In buffer material saturated with 0.01M carbonate aqueous solution, no passivation was observed and the effect of impurities on the anodic polarization behavior was small. (4) The corrosion rate of carbon steel in seawater was increased with the concentration of impurities. Among the impurity elements, the effects of P and Mn were relatively large. (5) It was inferred that the increase in corrosion rates in synthetic seawater by the addition of Si, Mn and P was promoted by the activation of hydrogen evolution reaction as a cathodic reaction.