Dynamics of water and formation mechanism of gas hydrates studied by neutron scattering under gas high-pressure
Kikuchi, Tatsuya 
 ; Matsumoto, Masakazu*; Yamamuro, Osamu*
; Matsumoto, Masakazu*; Yamamuro, Osamu*
We are studying the formation mechanism of gas hydrates currently attracting much attention in the research field of clathrate hydrates. The largest difficulty for this study is that guest gas molecules hardly dissolve into water under ambient pressure. In order to overcome this difficulty, we prepared aqueous solutions with high solubility (2% at maximum) of guest gases by applying gas high-pressure to water. The quasi-elastic neutron scatterings (QENS) of these samples have been measured to investigate the dynamics of water molecules affected by the guest molecules. The measurements were carried out on AGNES spectrometer installed at JRR-3 (JAEA, Tokai) and maintained by ISSP, University of Tokyo. The guest molecules taken in this study were Ar, Xe, N , and CO
, and CO which have simple molecular structures. The pressure and temperature ranges were 0-100 MPa and 263-363 K, respectively. We analyzed the QENS spectra
which have simple molecular structures. The pressure and temperature ranges were 0-100 MPa and 263-363 K, respectively. We analyzed the QENS spectra 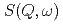 based on the jump diffusion model. The diffusion coefficient
based on the jump diffusion model. The diffusion coefficient  is smaller than
is smaller than  of pure water especially below the hydrate-formation temperature
of pure water especially below the hydrate-formation temperature 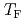 and for the guest molecules with high solubility. These experimental results were reproduced well by MD simulations. It was also found that the gas molecules get closer to each other and the diffusion of water molecules near gas molecules is suppressed, resulting in the smaller diffusion coefficient below
and for the guest molecules with high solubility. These experimental results were reproduced well by MD simulations. It was also found that the gas molecules get closer to each other and the diffusion of water molecules near gas molecules is suppressed, resulting in the smaller diffusion coefficient below 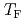 .
.