Isolation and characterization of the fragrant cyclamen  -methyltransferase involved in flower coloration
-methyltransferase involved in flower coloration
Akita, Yusuke; Kitamura, Satoshi; Hase, Yoshihiro; Narumi, Issei; Ishizaka, Hiroshi*; Kondo, Emiko*; Kameari, Naoko*; Nakayama, Masayoshi*; Tanikawa, Natsu*; Morita, Yasumasa*; Tanaka, Atsushi
Anthocyanin  -methyltransferase (OMT) is one of the key enzymes for anthocyanin modification and flower pigmentation. We previously bred a novel red-purple-flowered fragrant cyclamen (KMrp) from the purple-flowered fragrant cyclamen "Kaori-no-mai" (KM) by ion-beam irradiation. Since the major anthocyanins in KMrp and KM petals were delphinidin 3,5-diglucoside and malvidin 3,5-diglucoside, respectively, inactivation of a methylation step in the anthocyanin biosynthetic pathway was indicated in KMrp. We isolated and compared
-methyltransferase (OMT) is one of the key enzymes for anthocyanin modification and flower pigmentation. We previously bred a novel red-purple-flowered fragrant cyclamen (KMrp) from the purple-flowered fragrant cyclamen "Kaori-no-mai" (KM) by ion-beam irradiation. Since the major anthocyanins in KMrp and KM petals were delphinidin 3,5-diglucoside and malvidin 3,5-diglucoside, respectively, inactivation of a methylation step in the anthocyanin biosynthetic pathway was indicated in KMrp. We isolated and compared 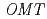 genes expressed in KM and KMrp petals. RT-PCR analysis revealed that
genes expressed in KM and KMrp petals. RT-PCR analysis revealed that 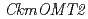 was expressed in the petals of KM but not in KMrp. Three additional
was expressed in the petals of KM but not in KMrp. Three additional 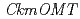 s with identical sequences were expressed in petals of both KM and KMrp. Genomic PCR analysis revealed that
s with identical sequences were expressed in petals of both KM and KMrp. Genomic PCR analysis revealed that 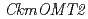 was not amplified from the KMrp genome, indicating that ion-beam irradiation caused a loss of the entire
was not amplified from the KMrp genome, indicating that ion-beam irradiation caused a loss of the entire 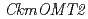 region in KMrp. In vitro enzyme assay demonstrated that CkmOMT2 catalyzes the 3' or 3',5'
region in KMrp. In vitro enzyme assay demonstrated that CkmOMT2 catalyzes the 3' or 3',5'  -methylation of the B-ring of anthocyanin substrates. These results suggest that CkmOMT2 is functional for anthocyanin methylation, and defective expression of
-methylation of the B-ring of anthocyanin substrates. These results suggest that CkmOMT2 is functional for anthocyanin methylation, and defective expression of 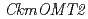 is responsible for changes in anthocyanin composition and flower coloration in KMrp.
is responsible for changes in anthocyanin composition and flower coloration in KMrp.