Formation of NaCl-type monodeuteride LaD by the disproportionation reaction of LaD
Machida, Akihiko; Honda, Mitsunori*; Hattori, Takanori 

 ; Sano, Asami
; Sano, Asami 

 ; Watanuki, Tetsu; Katayama, Yoshinori; Aoki, Katsutoshi; Komatsu, Kazuki*; Arima, Hiroshi*; Oshita, Hidetoshi*; Ikeda, Kazutaka*; Suzuya, Kentaro; Otomo, Toshiya*; Tsubota, Masami*; Doi, Koichi*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Kim, D. Y.*
; Watanuki, Tetsu; Katayama, Yoshinori; Aoki, Katsutoshi; Komatsu, Kazuki*; Arima, Hiroshi*; Oshita, Hidetoshi*; Ikeda, Kazutaka*; Suzuya, Kentaro; Otomo, Toshiya*; Tsubota, Masami*; Doi, Koichi*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Kim, D. Y.*
Hydrogen atoms absorbed in a metal occupy the interstitial sites of the metal lattice. In an fcc metal lattice, each metal atom has two tetrahedral (T) and one octahedral (O) sites that can accommodate hydrogen. Rare-earth metal La forms T-site occupied LaH and fully occupied LaH
and fully occupied LaH . O-site occupied or NaCl-type monohydride has yet to be reported for rare-earth metals. Previous X-ray diffraction measurements revealed the pressure-induced decomposition of an fcc-LaH
. O-site occupied or NaCl-type monohydride has yet to be reported for rare-earth metals. Previous X-ray diffraction measurements revealed the pressure-induced decomposition of an fcc-LaH into H-rich and H-poor phases around 11 GPa. The present neutron diffraction measurements on LaD
into H-rich and H-poor phases around 11 GPa. The present neutron diffraction measurements on LaD confirm the formation of NaCl-type LaD as a counterpart of the D-rich LaD
confirm the formation of NaCl-type LaD as a counterpart of the D-rich LaD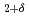 by disproportionation. First-principle calculations demonstrate that the NaCl-type LaH is stabilized at high pressures. Finding the NaCl-type LaH will pave the way for investigations on the site-dependent nature of hydrogen-metal interactions.
by disproportionation. First-principle calculations demonstrate that the NaCl-type LaH is stabilized at high pressures. Finding the NaCl-type LaH will pave the way for investigations on the site-dependent nature of hydrogen-metal interactions.