Selective separation of samarium(III) by synergistic extraction with  -diketone and methylphenylphenanthroline carboxamide
-diketone and methylphenylphenanthroline carboxamide
メチルフェニルフェナントロリンと -ジケトンとの共同効果によるサマリウム(III)の共同抽出
-ジケトンとの共同効果によるサマリウム(III)の共同抽出
長谷川 裕子*; 玉城 沙也香*; 矢島 弘文*; 橋本 文治*; 矢板 毅
Hasegawa, Yuko*; Tamaki, Sayaka*; Yajima, Hirofumi*; Hashimoto, Bunji*; Yaita, Tsuyoshi
Synergistic extraction of trivalent lanthanides (Lns(III)) with pivaloyltrifluoroacetone (HA) and N-methyl-N-phenyl-1,10-phenanthroline-2-carboxamide (MePhPTA) was evaluated across the Ln series. The distribution ratio ( ) of Sm(III) under an identical condition was the largest among all Lns(III). The separation factor (SF) between Sm(III) and Nd(III) (SF=
) of Sm(III) under an identical condition was the largest among all Lns(III). The separation factor (SF) between Sm(III) and Nd(III) (SF=
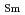 /
/
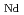 ) was 2.0 and SF between Sm(III) and Eu(III), (
) was 2.0 and SF between Sm(III) and Eu(III), (
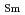 /
/
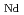 ) was 1.4. Upon analyzing the extraction data in detail on the basis of mass balance, it was found that the dominant extracted species of light Lns(III) was a stable ternary complex consisting of Ln(III), HA, and MePhPTA (B), namely, LnA
) was 1.4. Upon analyzing the extraction data in detail on the basis of mass balance, it was found that the dominant extracted species of light Lns(III) was a stable ternary complex consisting of Ln(III), HA, and MePhPTA (B), namely, LnA B, while the dominant extracted species of heavy Lns(III) was the ion pair, [LnA
B, while the dominant extracted species of heavy Lns(III) was the ion pair, [LnA B]
B] ClO
ClO
 . The complex for Pr(III) was very stable. It suggests that LnA
. The complex for Pr(III) was very stable. It suggests that LnA can form two 5-membered rings with MePhPTA, and the size of Pr(III) matches to the distance between the donor atoms in MePhPTA.
can form two 5-membered rings with MePhPTA, and the size of Pr(III) matches to the distance between the donor atoms in MePhPTA.