Extraction behavior of hexavalent Mo and W for Sg experiment
Miyashita, Sunao; Oe, Kazuhiro; Toyoshima, Atsushi; Sato, Tetsuya 

 ; Asai, Masato
; Asai, Masato 
 ; Tsukada, Kazuaki
; Tsukada, Kazuaki 
 ; Nagame, Yuichiro
; Nagame, Yuichiro  ; Sch
; Sch del, M.; Kaneya, Yusuke*; Omtvedt, J. P.*; Kratz, J. V.*; Haba, Hiromitsu*; Wada, Ayaka*; Kitayama, Yuta*
del, M.; Kaneya, Yusuke*; Omtvedt, J. P.*; Kratz, J. V.*; Haba, Hiromitsu*; Wada, Ayaka*; Kitayama, Yuta*
For the experiments of the redox potentials of Sg, rapid separation between different oxidation states of Sg is needed. In this study, solvent extraction of hexavalent Mo and W, as the lighter homologues of Sg, from 1.0 mol/L hydrochloric acid solution into di-(2-ethylhexyl)phosphoric acid (HDEHP), 1-phenyl-3-metyl-4-benzoyl-5-pyrazolone (PMBP), N-benzoyl-N-pheny-hydroxylamine (BPHA) and 4-isopropyl-tropolone (HT) in toluene was carried out. When HDEHP and PMBP were used as extractant, extraction equilibrium of  W was achieved within 5 and 1 hours respectively. On the other hand, when BPHA and HT were used, extraction equilibrium of
W was achieved within 5 and 1 hours respectively. On the other hand, when BPHA and HT were used, extraction equilibrium of  W was quickly achieved within 1 minute in both extractants. Extraction kinetics of
W was quickly achieved within 1 minute in both extractants. Extraction kinetics of 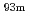 Mo using BPHA and HT was examined. Extraction kinetics of Mo was slower than that of W. Extraction equilibrium of Mo was achieved within 10 by BPHA and 3 minutes by HT. We concluded that HT is a suitable extractant for the Sg reduction experiment.
Mo using BPHA and HT was examined. Extraction kinetics of Mo was slower than that of W. Extraction equilibrium of Mo was achieved within 10 by BPHA and 3 minutes by HT. We concluded that HT is a suitable extractant for the Sg reduction experiment.