Crystal structures of the catalytic domain of a novel chitinase belonging to GH family 23
Arimori, Takao*; Kawamoto, Noriko*; Okazaki, Nobuo*; Nakazawa, Masami*; Miyatake, Kazutaka*; Fukamizo, Tamo*; Ueda, Mitsuhiro*; Tamada, Taro
Chitin, linear  -1,4-linked polymer of
-1,4-linked polymer of  -acetyl-D-glucosamine (NAG), is the second abundant biopolymer in nature next to cellulose. Hydrolysis of chitin provides useful products,
-acetyl-D-glucosamine (NAG), is the second abundant biopolymer in nature next to cellulose. Hydrolysis of chitin provides useful products,  -acetyl-chitooligosaccharides [(NAG)
-acetyl-chitooligosaccharides [(NAG) ] and chitooligosaccharides, which have a variety of biological functions including antibacterial activity and antitumor activity. We have previously cloned a novel chitinase gene from a moderate thermophilic strain
] and chitooligosaccharides, which have a variety of biological functions including antibacterial activity and antitumor activity. We have previously cloned a novel chitinase gene from a moderate thermophilic strain 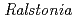 sp. A-471 (Ra-ChiC). Ra-ChiC comprises a signal peptide, a chitin-binding domain, an interdomain linker, and a catalytic domain. The catalytic domain shares amino acid sequence homology with goose type (G-type) lysozymes and, unlike other chitinases, Ra-ChiC belongs to glycohydrolase (GH) family 23. However, Ra-ChiC does not exhibit lysozyme activity, but only chitinase activity. In this study, we aim to reveal how Ra-ChiC catalyzes the hydrolysis of chitin and why Ra-ChiC exhibits chitinase activity instead of lysozyme activity. We determined the crystal structures of the catalytic domain of Ra-ChiC (Ra-ChiC
sp. A-471 (Ra-ChiC). Ra-ChiC comprises a signal peptide, a chitin-binding domain, an interdomain linker, and a catalytic domain. The catalytic domain shares amino acid sequence homology with goose type (G-type) lysozymes and, unlike other chitinases, Ra-ChiC belongs to glycohydrolase (GH) family 23. However, Ra-ChiC does not exhibit lysozyme activity, but only chitinase activity. In this study, we aim to reveal how Ra-ChiC catalyzes the hydrolysis of chitin and why Ra-ChiC exhibits chitinase activity instead of lysozyme activity. We determined the crystal structures of the catalytic domain of Ra-ChiC (Ra-ChiC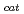 ), Ra-ChiC
), Ra-ChiC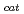 complexed with (NAG)
complexed with (NAG) , E141Q mutant of Ra-ChiC
, E141Q mutant of Ra-ChiC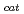 complexed with (NAG)
complexed with (NAG) , E162Q mutant of Ra-ChiC
, E162Q mutant of Ra-ChiC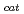 , and E162Q mutant of Ra-ChiC
, and E162Q mutant of Ra-ChiC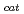 complexed with (NAG)
complexed with (NAG) . These structures provided us structural basis of substrate recognition mechanism and revealed that Ra-ChiC has a unique substrate-binding site including a tunnel-shaped cavity, which determines the substrate specificity. In addition, we also carried out a mutation analysis of acidic amino acid residues located at the active site. As a result, we found that not only a highly conserved Glu141 but also Asp226 located at the roof of the tunnel have quite important roles in catalysis.
. These structures provided us structural basis of substrate recognition mechanism and revealed that Ra-ChiC has a unique substrate-binding site including a tunnel-shaped cavity, which determines the substrate specificity. In addition, we also carried out a mutation analysis of acidic amino acid residues located at the active site. As a result, we found that not only a highly conserved Glu141 but also Asp226 located at the roof of the tunnel have quite important roles in catalysis.