Evaluation of Gibbs free energies of formation of Ce-Cd intermetallic compounds using electrochemical techniques
Shibata, Hiroki  ; Hayashi, Hirokazu
; Hayashi, Hirokazu  ; Akabori, Mitsuo; Arai, Yasuo; Kurata, Masaki
; Akabori, Mitsuo; Arai, Yasuo; Kurata, Masaki 
Gibbs free energies of formation of six Ce-Cd intermetallic compounds, CeCd, CeCd , CeCd
, CeCd , CeCd
, CeCd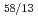 , CeCd
, CeCd and CeCd
and CeCd , were evaluated systematically using electrochemical techniques in the temperature range from 673 to 923 K in the LiCl-KCl-CeCl
, were evaluated systematically using electrochemical techniques in the temperature range from 673 to 923 K in the LiCl-KCl-CeCl -CdCl
-CdCl molten salt bath. The linear dependence of the Gibbs free energies of formation on temperature yields to the enthalpies and entropies of formation of these intermetallic compounds. By extrapolating the molar Gibbs free energy of Ce-Cd intermetallic compounds to the Cd distillation temperature, it was clear that the molar Gibbs free energy of Ce in Ce-Cd intermetallic compounds decreases gradually from CeCd
molten salt bath. The linear dependence of the Gibbs free energies of formation on temperature yields to the enthalpies and entropies of formation of these intermetallic compounds. By extrapolating the molar Gibbs free energy of Ce-Cd intermetallic compounds to the Cd distillation temperature, it was clear that the molar Gibbs free energy of Ce in Ce-Cd intermetallic compounds decreases gradually from CeCd to CeCd
to CeCd and attains to the minimum value at CeCd
and attains to the minimum value at CeCd . This suggests on the Cd distillation from the U-Pu-Ce-Cd alloy that the dissolution of U or Pu into CeCd
. This suggests on the Cd distillation from the U-Pu-Ce-Cd alloy that the dissolution of U or Pu into CeCd should be mostly taken into consideration.
should be mostly taken into consideration.