Optical properties of tetravalent uranium complexes in non-aqueous media
Aoyagi, Noboru 

 ; Watanabe, Masayuki
; Watanabe, Masayuki 

 ; Kirishima, Akira*; Sato, Nobuaki*; Kimura, Takaumi
; Kirishima, Akira*; Sato, Nobuaki*; Kimura, Takaumi 
Tetravalent uranium halides were studied to understand photo-physical behaviors under the unexplored condition such as in non-aqueous media at ambient condition or at a cryogenic temperature. The anhydrous uranium tetrahalides (synthesized) were added into a series of dry organic solvents or a hydrophilic ionic liquid to observe their UV-Vis-NIR absorption spectra and time-resolved luminescence spectra in detail. The lower energy bands of UX (X = F, Br, and I) appearing in UV-Vis-NIR absorption spectra in dry 1,4-dioxane were assigned as the transition from the ground state manifold to higher multiples ranging from 6,000 to 12,000 cm
(X = F, Br, and I) appearing in UV-Vis-NIR absorption spectra in dry 1,4-dioxane were assigned as the transition from the ground state manifold to higher multiples ranging from 6,000 to 12,000 cm . In contrast, there is the spectral emerging for these absorption spectra in the IL at higher energy band around 12,000 to 18,000 cm
. In contrast, there is the spectral emerging for these absorption spectra in the IL at higher energy band around 12,000 to 18,000 cm with each peak broadened. These complexes exhibit white photoluminescence by UV-pulse excitation at
with each peak broadened. These complexes exhibit white photoluminescence by UV-pulse excitation at 
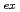 = 394 nm, with broad multiple peaks assigned. The fluorescence lifetimes for these are 12.8 ns (1,4-dioxane) and 18.6 ns (IL) in spite of the longer U-I separation than that of U-NCS. Chemical species and the coordination number of U
= 394 nm, with broad multiple peaks assigned. The fluorescence lifetimes for these are 12.8 ns (1,4-dioxane) and 18.6 ns (IL) in spite of the longer U-I separation than that of U-NCS. Chemical species and the coordination number of U in water, in organic solutions, and in an IL could be thoroughly different, resulting in a variety of spectra.
in water, in organic solutions, and in an IL could be thoroughly different, resulting in a variety of spectra.