Ultra-trace analysis of plutonium by thermal ionization mass spectrometry with a continuous heating technique without chemical separation
Lee, C.-G.*; Suzuki, Daisuke 

 ; Esaka, Fumitaka
; Esaka, Fumitaka 
 ; Magara, Masaaki
; Magara, Masaaki 
 ; Song, K.*
; Song, K.*
Thermal ionization mass spectrometry (TIMS) with a continuous heating technique is known as an effective method for measuring the isotope ratio in trace amounts of uranium. In this study, the analytical performance of thermal ionization mass spectrometry with a continuous heating technique was investigated using a standard plutonium solution (SRM 947). The influence of the heating rate of the evaporation filament on the precision and accuracy of the isotope ratios was examined using a plutonium solution sample at the fg level. Changing the heating rate of the evaporation filament on samples ranging from 0.1 fg to 1000 fg revealed that the influence of the heating rate on the precision and accuracy of the isotope ratios was slight around the heating rate range of 100 to 250 mA/min. All of the isotope ratios of plutonium (SRM 947),  Pu/
Pu/ Pu,
Pu,  Pu/
Pu/ Pu,
Pu,  Pu/
Pu/ Pu and
Pu and 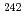 Pu/
Pu/ Pu, were measured down to sample amounts of 70 fg. The ratio of
Pu, were measured down to sample amounts of 70 fg. The ratio of  Pu/
Pu/ Pu was measured down to a sample amount of 0.1 fg, which corresponds to a PuO
Pu was measured down to a sample amount of 0.1 fg, which corresponds to a PuO particle with a diameter of 0.2
particle with a diameter of 0.2  m. Moreover, the signals of
m. Moreover, the signals of  Pu could be detected with a sample amount of 0.03 fg, which corresponds to the detection limit of
Pu could be detected with a sample amount of 0.03 fg, which corresponds to the detection limit of  Pu of 0.006 fg as estimated by the 3
Pu of 0.006 fg as estimated by the 3  criterion.
criterion.  Pu and
Pu and  Am formed by the decay of
Am formed by the decay of  Pu could be discriminated owing to the difference in the evaporation temperature. As a result,
Pu could be discriminated owing to the difference in the evaporation temperature. As a result,  Pu/
Pu/ Pu as well as
Pu as well as  Pu/
Pu/ Pu and
Pu and 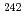 Pu/
Pu/ Pu in plutonium samples could be measured by TIMS with a continuous heating technique and without any chemical separation processes.
Pu in plutonium samples could be measured by TIMS with a continuous heating technique and without any chemical separation processes.