A Method of extraction equilibrium analysis in a synergistic ionic-liquid extraction system; Lanthanoids(III)-Htta-TOPO system
Okamura, Hiroyuki 

 ; Mizuno, Masayoshi*; Hirayama, Naoki*; Shimojo, Kojiro
; Mizuno, Masayoshi*; Hirayama, Naoki*; Shimojo, Kojiro 

 ; Naganawa, Hirochika
; Naganawa, Hirochika 
 ; Imura, Hisanori*
; Imura, Hisanori*
Solvent extraction technique is widely investigated to separate lanthanoids (Ln). The development of a more effective separation system is an important issue in the field of atomic energy. Recently, the synergistic ionic-liquid (IL) extraction of Ln(III) with several  -diketones and trioctylphosphine oxide (TOPO) in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide was studied, and it was found that the separability of Ln(III) was remarkably enhanced by the selective synergism for heavier rate earth. In this study, the method of extraction equilibrium analysis for Ln(III) in the synergistic ionic-liquid extraction system with 2-thenoyltrifluoroacetone (Htta) and TOPO was investigated. To determine the extraction constants of the respective Ln(III) complexes, a three-dimensional non-linear analysis of log
-diketones and trioctylphosphine oxide (TOPO) in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide was studied, and it was found that the separability of Ln(III) was remarkably enhanced by the selective synergism for heavier rate earth. In this study, the method of extraction equilibrium analysis for Ln(III) in the synergistic ionic-liquid extraction system with 2-thenoyltrifluoroacetone (Htta) and TOPO was investigated. To determine the extraction constants of the respective Ln(III) complexes, a three-dimensional non-linear analysis of log  -log [tta
-log [tta ]
]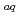 -log [TOPO]
-log [TOPO]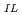 was applied. The formation constants of various ternary complexes formed in IL were calculated, and the order of the formation constants of the Ln(tta)
was applied. The formation constants of various ternary complexes formed in IL were calculated, and the order of the formation constants of the Ln(tta) (TOPO)
(TOPO)
 complexes was found to be Eu(III)
complexes was found to be Eu(III)  Nd(III). Consequently, it was suggested that the formation of hydrophobic charged complexes is a factor for the selective synergism.
Nd(III). Consequently, it was suggested that the formation of hydrophobic charged complexes is a factor for the selective synergism.