X-ray absorption fine structure at the cesium  3 absorption edge for cesium sorbed in clay minerals
3 absorption edge for cesium sorbed in clay minerals
Honda, Mitsunori 

 ; Shimoyama, Iwao
; Shimoyama, Iwao 

 ; Okamoto, Yoshihiro
; Okamoto, Yoshihiro 
 ; Baba, Yuji
; Baba, Yuji 
 ; Suzuki, Shinichi; Yaita, Tsuyoshi
; Suzuki, Shinichi; Yaita, Tsuyoshi
We present the use of near-edge X-ray absorption fine structure (NEXAFS) to investigate local electronic structures of cesium ions sorbed in two types of clay minerals (vermiculite and kaolinite) with a different capacity to fix Cs. NEXAFS is element specific because X-ray absorption edges of different elements have different energies. However, the energy of the Cs  3 absorption edge is close to that of the
3 absorption edge is close to that of the  -edge of titanium generally contained in clay minerals. Therefore, Cs
-edge of titanium generally contained in clay minerals. Therefore, Cs  3-edge NEXAFS measurements of Cs in clay minerals have not yet succeeded. In this study, we successfully measured pure Cs
3-edge NEXAFS measurements of Cs in clay minerals have not yet succeeded. In this study, we successfully measured pure Cs  3-edge NEXAFS spectra for cesium sorbed in clay minerals by completely separating Ti
3-edge NEXAFS spectra for cesium sorbed in clay minerals by completely separating Ti 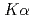 and Cs
and Cs 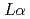 fluorescence X-rays using a fluorescence method. We confirmed the peak intensity between vermiculite and kaolinite in the NEXAFS spectra. To clarify the identification of NEXAFS spectra, theoretical calculations were performed using the discrete variational X
fluorescence X-rays using a fluorescence method. We confirmed the peak intensity between vermiculite and kaolinite in the NEXAFS spectra. To clarify the identification of NEXAFS spectra, theoretical calculations were performed using the discrete variational X molecular orbital method (DV-X
molecular orbital method (DV-X ), and peak identification was achieved. The difference in peak intensity was explained by the difference in the electron density of unoccupied molecular orbitals. We studied the influence of water molecules and found a change in the electron densities of unoccupied molecular orbitals caused by the coordination of water molecules.
), and peak identification was achieved. The difference in peak intensity was explained by the difference in the electron density of unoccupied molecular orbitals. We studied the influence of water molecules and found a change in the electron densities of unoccupied molecular orbitals caused by the coordination of water molecules.