Effect of substituents in 1,2,4-triazin-3-yl 1,10-phenanthroline on the extraction and complexation of Am and lanthanide series elements
and lanthanide series elements
Kamezawa, Akinori*; Suzuki, Shinichi; Kobayashi, Toru  ; Sueki, Keisuke*
; Sueki, Keisuke*
A solvent extraction method is one of the useful technique for the selective separation of minor actinides and lanthanides from high level radioactive waste. Therefore, it is required to advance the performance of an extraction agent. However, extraction mechanisms for the desirable separation ability are still unclear. In the present study, we focused on the certain ligands which contain tridentate coordination site as 2-(1,2,4-triazine-3-yl) 1,10-phenanthroline (TPhen). Partitioning experiments by such ligands were hardly investigated. We synthesized three TPhen ligands having different substituents (methyl group, ethyl group, phenyl group) of each triazine ring at 5,6-position. Three types of ligands were synthesized from 1,10-phenanthroline-2-carboxamidehydrazone with  -diketone following known procedures. All the ligands were obtained as hydrates identified by NMR and elementary analysis. All the reaction yields were 40
-diketone following known procedures. All the ligands were obtained as hydrates identified by NMR and elementary analysis. All the reaction yields were 40  50 %. The ligand protonation and complexation equilibria were determined by UV-Vis spectroscopic titrations with Eu
50 %. The ligand protonation and complexation equilibria were determined by UV-Vis spectroscopic titrations with Eu or H
or H in acetonitrile solution. The extraction experiments of americium and lanthanide series elements except for promethium were performed with changing the nitric acid concentration, the ligand concentration and the organic phase compositions. And then the distribution ratios of these elements between two phases and separation factors (SF
in acetonitrile solution. The extraction experiments of americium and lanthanide series elements except for promethium were performed with changing the nitric acid concentration, the ligand concentration and the organic phase compositions. And then the distribution ratios of these elements between two phases and separation factors (SF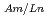 ) were determined.
) were determined.