Extraction equilibrium of lanthanoids(III) in ionic liquid synergistic extraction systems; Effect of  -diketones on the extracted species
-diketones on the extracted species
Okamura, Hiroyuki 

 ; Hatakeyama, Mizuki*; Nagatani, Hirohisa*; Nishiyama, Yoshio*; Shimojo, Kojiro
; Hatakeyama, Mizuki*; Nagatani, Hirohisa*; Nishiyama, Yoshio*; Shimojo, Kojiro 

 ; Naganawa, Hirochika
; Naganawa, Hirochika 
 ; Imura, Hisanori*
; Imura, Hisanori*
In the separation of lanthanoids (Lns), the synergistic extraction systems have been widely investigated to enhance the extractability and separability. Recently, a method of extraction equilibrium analysis for Ln(III) in the ionic liquid (IL) synergistic extraction system with 2-thenoyltrifluoroacetone (Htta)-trioctylphosphine oxide (TOPO) in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide was reported. In this study, the extraction equilibrium of Ln in the benzoylacetone (Hba)-TOPO system exhibiting higher Ln separability than the Htta-TOPO system was investigated. The effect of  -diketones on the extracted species and the relationship between the extracted species and Ln separability were clarified. A three-dimensional non-linear analysis of log
-diketones on the extracted species and the relationship between the extracted species and Ln separability were clarified. A three-dimensional non-linear analysis of log  -log [ba
-log [ba ]
]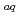 -log [TOPO]
-log [TOPO]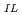 was applied in the Hba-TOPO synergistic extraction system, and both Ln(ba)
was applied in the Hba-TOPO synergistic extraction system, and both Ln(ba) (TOPO)
(TOPO)
 and Ln(ba)(TOPO)
and Ln(ba)(TOPO)
 were found as the extracted species. The formation of 1:1:4 dicationic complex was not observed in the Htta-TOPO synergistic extraction system, indicating that the extracted species is dependent on the acidity of
were found as the extracted species. The formation of 1:1:4 dicationic complex was not observed in the Htta-TOPO synergistic extraction system, indicating that the extracted species is dependent on the acidity of  -diketone. Consequently, it was suggested that the formation of the highly hydrophobic 1:1:4 complex is a factor of higher Ln separability.
-diketone. Consequently, it was suggested that the formation of the highly hydrophobic 1:1:4 complex is a factor of higher Ln separability.