Adsorption behavior of lawrencium (Lr) on a tantalum surface
Sato, Tetsuya 

 ; Kaneya, Yusuke*; Asai, Masato
; Kaneya, Yusuke*; Asai, Masato 
 ; Tsukada, Kazuaki
; Tsukada, Kazuaki 
 ; Toyoshima, Atsushi; Mitsukai, Akina*; Osa, Akihiko
; Toyoshima, Atsushi; Mitsukai, Akina*; Osa, Akihiko 
 ; Makii, Hiroyuki
; Makii, Hiroyuki 

 ; Nishio, Katsuhisa
; Nishio, Katsuhisa 

 ; Hirose, Kentaro
; Hirose, Kentaro 
 ; Nagame, Yuichiro
; Nagame, Yuichiro  ; Tomitsuka, Tomohiro*; Shirai, Kaori*; Sato, Daisuke*; Oe, Kazuhiro*; Goto, Shinichi*; Miyashita, Sunao*; Shingu, Kazutoshi*; Naguwa, Ryo*; Shibata, Michihiro*; Kamada, Hiroki*; Kasamatsu, Yoshitaka*; Shigekawa, Yudai*; Sakama, Minoru*; D
; Tomitsuka, Tomohiro*; Shirai, Kaori*; Sato, Daisuke*; Oe, Kazuhiro*; Goto, Shinichi*; Miyashita, Sunao*; Shingu, Kazutoshi*; Naguwa, Ryo*; Shibata, Michihiro*; Kamada, Hiroki*; Kasamatsu, Yoshitaka*; Shigekawa, Yudai*; Sakama, Minoru*; D llmann, Ch. E.*; Eberhardt, K.*; Grund, J.*; Kratz, J. V.*; Runke, J.*; Th
llmann, Ch. E.*; Eberhardt, K.*; Grund, J.*; Kratz, J. V.*; Runke, J.*; Th rle-Pospiech, P.*; Trautmann, N.*; Pershina, V.*; Sch
rle-Pospiech, P.*; Trautmann, N.*; Pershina, V.*; Sch del, M.*; Yakushev, A.*; Eichler, R.*; Steinegger, P.*
del, M.*; Yakushev, A.*; Eichler, R.*; Steinegger, P.*
The ground state electronic configuration of lawrencium (Lr, Z =103) is predicted to be [Rn]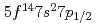 , which is different from that of the lanthanide homolog Lu [Xe]
, which is different from that of the lanthanide homolog Lu [Xe]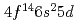 due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of
due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of  Lr, which was produced in the reaction of
Lr, which was produced in the reaction of  Cf(
Cf(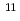 B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of
B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of  Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.
Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.