Adsorption of lawrencium (Lr) on a metallic tantalum (Ta) surface
Kaneya, Yusuke*; Tomitsuka, Tomohiro; Sato, Tetsuya 

 ; Asai, Masato
; Asai, Masato 
 ; Tsukada, Kazuaki
; Tsukada, Kazuaki 
 ; Toyoshima, Atsushi; Mitsukai, Akina; Makii, Hiroyuki
; Toyoshima, Atsushi; Mitsukai, Akina; Makii, Hiroyuki 

 ; Hirose, Kentaro
; Hirose, Kentaro 
 ; Osa, Akihiko
; Osa, Akihiko 
 ; Nishio, Katsuhisa
; Nishio, Katsuhisa 

 ; Nagame, Yuichiro
; Nagame, Yuichiro  ; Sato, Daisuke*; Shirai, Kaori*; Oe, Kazuhiro*; Goto, Shinichi*; Sakama, Minoru*; Naguwa, Ryo*; Shingu, Kazutoshi*; Miyashita, Sunao*; Kamada, Hiroki*; Shibata, Michihiro*; Shigekawa, Yudai*; Kasamatsu, Yoshitaka*; Steinegger, P.*; Eichler, R.*; Grund, J.*; Eberhardt, K.*; Runke, J.*; Th
; Sato, Daisuke*; Shirai, Kaori*; Oe, Kazuhiro*; Goto, Shinichi*; Sakama, Minoru*; Naguwa, Ryo*; Shingu, Kazutoshi*; Miyashita, Sunao*; Kamada, Hiroki*; Shibata, Michihiro*; Shigekawa, Yudai*; Kasamatsu, Yoshitaka*; Steinegger, P.*; Eichler, R.*; Grund, J.*; Eberhardt, K.*; Runke, J.*; Th rle-Pospiech, P.*; D
rle-Pospiech, P.*; D llmann, Ch. E.*; Trautmann, N.*; Kratz, J. V.*; Yakushev, A.*; Pershina, V.*; Sch
llmann, Ch. E.*; Trautmann, N.*; Kratz, J. V.*; Yakushev, A.*; Pershina, V.*; Sch del, M.*
del, M.*
Recently, we determined the first ionization potential of the heaviest actinide, lawrencium (Lr, 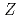 = 103), using a surface ion-source coupled to the Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator. The obtained value was in good agreement with that predicted by state-of-the-art relativistic calculations. This suggests that the outermost electron of the Lr atom is bound in a 7p
= 103), using a surface ion-source coupled to the Isotope Separator On-Line (ISOL) at the JAEA tandem accelerator. The obtained value was in good agreement with that predicted by state-of-the-art relativistic calculations. This suggests that the outermost electron of the Lr atom is bound in a 7p orbital, although a 6d orbital is anticipated to be occupied simply from the analogy to its lighter homologue lutetium (Lu) where a 5d orbital is occupied. This result motivates us to explore the volatility of elemental Lr because the adsorption enthalpy of Lr is expected to be indicative of the type of its interaction with a surface material. In the present work, the adsorption behavior of Lr is studied by a newly developed method combining vacuum chromatography with surface ionization in a metallic column/ionizer of the ISOL.
orbital, although a 6d orbital is anticipated to be occupied simply from the analogy to its lighter homologue lutetium (Lu) where a 5d orbital is occupied. This result motivates us to explore the volatility of elemental Lr because the adsorption enthalpy of Lr is expected to be indicative of the type of its interaction with a surface material. In the present work, the adsorption behavior of Lr is studied by a newly developed method combining vacuum chromatography with surface ionization in a metallic column/ionizer of the ISOL.