Behavior of intermolecular interactions in  -glycine under high pressure
-glycine under high pressure
Shinozaki, Ayako*; Komatsu, Kazuki*; Kagi, Hiroyuki*; Fujimoto, Chikako*; Machida, Shinichi*; Sano, Asami 

 ; Hattori, Takanori
; Hattori, Takanori 


Pressure-response on the crystal structure of deuterated  -glycine was investigated at room temperature, using powder and single-crystal X-ray diffraction, and powder neutron diffraction measurements under high pressure. No phase change was observed up to 8.7 GPa, although anisotropy of the lattice compressibility was found. Neutron diffraction measurements indicated the distance of the intermolecular D
-glycine was investigated at room temperature, using powder and single-crystal X-ray diffraction, and powder neutron diffraction measurements under high pressure. No phase change was observed up to 8.7 GPa, although anisotropy of the lattice compressibility was found. Neutron diffraction measurements indicated the distance of the intermolecular D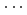 O bond along with the
O bond along with the 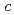 -axis increase with compression up to 6.4 GPa. The distance of another D
-axis increase with compression up to 6.4 GPa. The distance of another D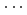 O bond along with the
O bond along with the 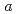 -axis decreased with increasing pressure, and became the shortest intermolecular hydrogen bond above 3 GPa. In contrast, the lengths of the bifurcated N-D
-axis decreased with increasing pressure, and became the shortest intermolecular hydrogen bond above 3 GPa. In contrast, the lengths of the bifurcated N-D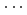 O and C-D
O and C-D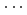 O hydrogen bonds, which are formed between the layers of the
O hydrogen bonds, which are formed between the layers of the  -glycine molecules along the
-glycine molecules along the 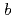 -axis, decreased significantly with increasing pressure. The decrease of the intermolecular distances resulted in the largest compressibility of the
-axis, decreased significantly with increasing pressure. The decrease of the intermolecular distances resulted in the largest compressibility of the 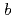 -axis, compared to the other two axes. Hirshfeld analysis suggested that the reduction of the void region size, rather than shrinkage of the strong N-D
-axis, compared to the other two axes. Hirshfeld analysis suggested that the reduction of the void region size, rather than shrinkage of the strong N-D O hydrogen bonds, occurred with compression.
O hydrogen bonds, occurred with compression.