Extension of the classical equilibrium analysis based on precise structure analysis of clustering phenomena by metal complexes
Okamura, Hiroyuki 

 ; Ueda, Yuki
; Ueda, Yuki 

 ; Motokawa, Ryuhei
; Motokawa, Ryuhei 

 ; Mu, J.*; Masters, A. J.*; Antonio, M. R.*
; Mu, J.*; Masters, A. J.*; Antonio, M. R.*
Under practical liquid-liquid extraction conditions with high solute concentrations, clusters of metal-extractant complexes can form in the organic phase. In these conditions, application of the slope analysis method for determining metal-extractant stoichiometry becomes difficult, and the deviations from ideal appear as non-linear responses and non-integer slopes. In this study, we developed novel extraction equilibrium analysis for practical liquid-liquid systems with consideration of the cluster formation and investigated the extension of the classical equilibrium analysis. Molecular dynamics (MD) simulation snapshots indicate the formation of aggregated clusters of 1 to 9 Zr(NO )
) (TBP)
(TBP) complexes in
complexes in  -octane, leading to the determination of composition and molar fraction of each cluster. Considering the extraction equilibrium of clusters composed of
-octane, leading to the determination of composition and molar fraction of each cluster. Considering the extraction equilibrium of clusters composed of 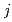 Zr(NO
Zr(NO )
) (TBP)
(TBP) complexes, the extraction equilibrium constants K
complexes, the extraction equilibrium constants K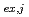 (
(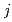 = 1-9) were calculated from the distribution ratio and the molar fraction for each cluster obtained by the MD analysis. The distribution curve calculated from the obtained K
= 1-9) were calculated from the distribution ratio and the molar fraction for each cluster obtained by the MD analysis. The distribution curve calculated from the obtained K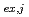 values agrees well with the experimental values. Therefore, MD simulation can accurately reproduce the experimental values in the clustering/aggregation liquid-liquid extraction system, which enabled us to extend the classical equilibrium analysis.
values agrees well with the experimental values. Therefore, MD simulation can accurately reproduce the experimental values in the clustering/aggregation liquid-liquid extraction system, which enabled us to extend the classical equilibrium analysis.