Leaching behavior of simulated fuel debris in the UO -SUS system prepared by irradiation or tracer doping method
-SUS system prepared by irradiation or tracer doping method
Sasaki, Takayuki*; Tonna, Ryutaro*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta 

 ; Kusaka, Ryoji
; Kusaka, Ryoji 
 ; Watanabe, Masayuki
; Watanabe, Masayuki 


Under the high-temperature conditions in the reactor cores of Fukushima Daiichi Nuclear Power Station (FDNPS) during the accident, UO , zircaloy, and structural materials such as stainless steel are thought to be reacted. Since it will take a long time to retrieve the fuel debris, it is essential to accumulate basic knowledge for anticipating the secular change of chemical properties. In this study, to examine the dissolution behavior to water, the simulated alloy-based debris samples were prepared by two methods, and the dissolution behavior was analyzed; 1) Irradiation method: the simulated debris was irradiated by thermal neutrons to introduce FP, 2) Doping method: non-radioactive elements (cold FPs) simulating FPs were doped to the sample. Based on the concentration
, zircaloy, and structural materials such as stainless steel are thought to be reacted. Since it will take a long time to retrieve the fuel debris, it is essential to accumulate basic knowledge for anticipating the secular change of chemical properties. In this study, to examine the dissolution behavior to water, the simulated alloy-based debris samples were prepared by two methods, and the dissolution behavior was analyzed; 1) Irradiation method: the simulated debris was irradiated by thermal neutrons to introduce FP, 2) Doping method: non-radioactive elements (cold FPs) simulating FPs were doped to the sample. Based on the concentration 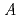 of the nuclide M in the sample, the leaching ratio
of the nuclide M in the sample, the leaching ratio 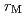 was evaluated from the relationship of
was evaluated from the relationship of 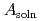 /
/ 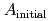 . The leaching ratio
. The leaching ratio 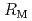 which was normalized by
which was normalized by 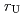 was also discussed. In samples of U
was also discussed. In samples of U O
O , U
, U Zr
Zr O
O and UCr(Fe)O
and UCr(Fe)O , Cs leached preferentially to U immediately after immersion (
, Cs leached preferentially to U immediately after immersion (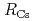
 ) in both the irradiation and the doping methods, and then the
) in both the irradiation and the doping methods, and then the 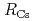 value decreased with time, suggesting U dissolution would be a rate-limiting reaction thereafter. Divalent Ba (FP) and Sr (cold FP) also leached preferentially to U, while trivalent Nd (FP) and Eu (cold FP) showed a harmonious dissolution with U. The
value decreased with time, suggesting U dissolution would be a rate-limiting reaction thereafter. Divalent Ba (FP) and Sr (cold FP) also leached preferentially to U, while trivalent Nd (FP) and Eu (cold FP) showed a harmonious dissolution with U. The 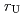 value was in the order of pure water (PW) and 0.1 M NaClO
value was in the order of pure water (PW) and 0.1 M NaClO (Na)
(Na)  artificial seawater (SW), and the effect of complex formation with anions in the solution on the order was observed. The
artificial seawater (SW), and the effect of complex formation with anions in the solution on the order was observed. The  values depended on the valence of ions; PW and Na
values depended on the valence of ions; PW and Na  SW for
SW for 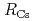 and
and 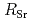 , while PW
, while PW  Na and SWfor
Na and SWfor 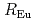 . The leaching behavior will be discussed in relation to the existing state in the solid phase and the chemical state in the aqueous phase.
. The leaching behavior will be discussed in relation to the existing state in the solid phase and the chemical state in the aqueous phase.