Diffusion of tritiated water,  Cs
Cs , and
, and 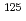 I
I in compacted Ca-montmorillonite; Experimental and modeling approaches
in compacted Ca-montmorillonite; Experimental and modeling approaches
Fukatsu, Yuta 
 ; Yotsuji, Kenji*; Okubo, Takahiro*; Tachi, Yukio
; Yotsuji, Kenji*; Okubo, Takahiro*; Tachi, Yukio 

Mechanistic understanding and predictive modeling of radionuclide diffusion in Na- and Ca-montmorillonite are essential to evaluate the long-term evolution of the bentonite barrier and their impact on radionuclide migration during geological disposal of radioactive wastes. Thus, the diffusion behavior of  Cs
Cs ,
, 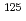 I
I , and tritiated water (HTO) in compacted Ca-montmorillonite was investigated as a function of porewater salinity and dry density via both experiments and models. The effective diffusion coefficient (De) followed in the order of
, and tritiated water (HTO) in compacted Ca-montmorillonite was investigated as a function of porewater salinity and dry density via both experiments and models. The effective diffusion coefficient (De) followed in the order of  Cs
Cs
 HTO
HTO 
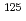 I
I . The De of
. The De of  Cs
Cs decreased with increasing salinity, whereas the dependence of De of
decreased with increasing salinity, whereas the dependence of De of 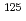 I
I on salinity was uncertain. The cation excess and anion exclusion effects for Ca-montmorillonite were lower than those for Na-montmorillonite. The integrated sorption and diffusion (ISD) model, assuming the homogeneous pore structure and the electrical double layer (EDL) theory for 2:1 electrolyte (CaCl
on salinity was uncertain. The cation excess and anion exclusion effects for Ca-montmorillonite were lower than those for Na-montmorillonite. The integrated sorption and diffusion (ISD) model, assuming the homogeneous pore structure and the electrical double layer (EDL) theory for 2:1 electrolyte (CaCl ), could account for the observed trends for De in Ca-montmorillonite. The lower dependence of De on the porewater salinity in Ca-montmorillonite was caused by the reduction of the EDL thickness for divalent cations (Ca
), could account for the observed trends for De in Ca-montmorillonite. The lower dependence of De on the porewater salinity in Ca-montmorillonite was caused by the reduction of the EDL thickness for divalent cations (Ca ) in comparison with that for monovalent cations (Na
) in comparison with that for monovalent cations (Na ). The multipore model could improve the fit for De of
). The multipore model could improve the fit for De of 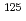 I
I at low salinity due to the reduction of interlayer pore volumes and anion exclusion effect, however, the disparity at higher densities was considerably larger. From these results, cation diffusion for compacted Ca-montmorillonite could be mainly explained by the electrostatic interactions in the homogeneous pore model; in contrast, anion diffusion was sensitive to both electrostatic interactions and heterogeneous pore structures. The proposed ISD model is an effective tool to evaluate the radionuclide diffusion and sorption behavior in both compacted Ca-montmorillonite and Na-montmorillonite.
at low salinity due to the reduction of interlayer pore volumes and anion exclusion effect, however, the disparity at higher densities was considerably larger. From these results, cation diffusion for compacted Ca-montmorillonite could be mainly explained by the electrostatic interactions in the homogeneous pore model; in contrast, anion diffusion was sensitive to both electrostatic interactions and heterogeneous pore structures. The proposed ISD model is an effective tool to evaluate the radionuclide diffusion and sorption behavior in both compacted Ca-montmorillonite and Na-montmorillonite.