Unusual redox behavior of ruthenocene confined in the micropores of activated carbon
活性炭細孔中ルテノセンの特異的酸化還元挙動
糸井 弘行*; Ninomiya, Takeru*; 長谷川 英之*; Maki, Shintaro*; Sakakibara, Akihiro*; 鈴木 隆太郎*; 笠井 湧斗*; 岩田 博之*; 松村 大樹 

 ; 大和田 真生*; 西原 洋知*; 大澤 善美*
; 大和田 真生*; 西原 洋知*; 大澤 善美*
Itoi, Hiroyuki*; Ninomiya, Takeru*; Hasegawa, Hideyuki*; Maki, Shintaro*; Sakakibara, Akihiro*; Suzuki, Ryutaro*; Kasai, Yuto*; Iwata, Hiroyuki*; Matsumura, Daiju; Owada, Mao*; Nishihara, Hirotomo*; Ozawa, Yoshimi*
We demonstrate reversible charge/discharge in ruthenocene, RuCp (Cp =
(Cp = 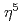 -C
-C H
H ), using activated carbon (AC) as a support. Upon subsequent electrochemical oxidation using an aqueous H
), using activated carbon (AC) as a support. Upon subsequent electrochemical oxidation using an aqueous H SO
SO electrolyte, the clusters are disassembled and the RuCp
electrolyte, the clusters are disassembled and the RuCp molecules are finely dispersed in the micropores. The resulting RuCp
molecules are finely dispersed in the micropores. The resulting RuCp has a large contact area with conductive carbon surfaces, thereby realizing rapid charge transfer at the contact interface. Consequently, rapid charge storage occurs via the reversible redox reaction of the supported RuCp
has a large contact area with conductive carbon surfaces, thereby realizing rapid charge transfer at the contact interface. Consequently, rapid charge storage occurs via the reversible redox reaction of the supported RuCp in aqueous H
in aqueous H SO
SO without dimerization or disproportionation reactions, which is confirmed by X-ray absorption spectroscopy. Since hybridization can produce different properties of the host and guest materials, their infinite combinations would have the possibility to yield properties far surpassing those of existing materials.
without dimerization or disproportionation reactions, which is confirmed by X-ray absorption spectroscopy. Since hybridization can produce different properties of the host and guest materials, their infinite combinations would have the possibility to yield properties far surpassing those of existing materials.