Microscopic dynamics of lithium diffusion in single crystal of the solid-state electrolyte La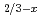 Li
Li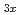 TiO
TiO (x = 0.13) studied by quasielastic neutron scattering
(x = 0.13) studied by quasielastic neutron scattering
Matsuura, Masato*; Fujiwara, Yasuyuki*; Moriwake, Hiroki*; Ohara, Koji*; Kawakita, Yukinobu 

Quasielastic neutron scattering (QENS) measurements combined with first principles based molecular dynamics calculations were conducted to study the dynamics of Li ions in a solid-state electrolyte La
ions in a solid-state electrolyte La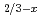 Li
Li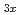 TiO
TiO (LLTO) with
(LLTO) with 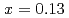 . By using a large
. By using a large  Li-enriched single crystal sample, a QENS signal was clearly observed along the three principal axes [110], [111], and [001] at a temperature (
Li-enriched single crystal sample, a QENS signal was clearly observed along the three principal axes [110], [111], and [001] at a temperature ( ) of 600 K. Wave vector dependence of the linewidth of the QENS signal along each direction was explained well using the Chudley-Elliot model for jumps between the A sites of the perovskite lattice through the bottleneck square, which was also supported by molecular dynamics calculations. The estimated self-diffusion coefficient of Li
) of 600 K. Wave vector dependence of the linewidth of the QENS signal along each direction was explained well using the Chudley-Elliot model for jumps between the A sites of the perovskite lattice through the bottleneck square, which was also supported by molecular dynamics calculations. The estimated self-diffusion coefficient of Li (
(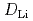 ) in the ab plane was slightly larger than that along the c axis, suggesting quasi-isotropic diffusion, that is, the three-dimensional diffusion of Li
) in the ab plane was slightly larger than that along the c axis, suggesting quasi-isotropic diffusion, that is, the three-dimensional diffusion of Li ions. The decrease in
ions. The decrease in 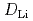 with decreasing
with decreasing  was reasonably explained by a thermal activation process with the activation energy determined from ionic-conductivity measurements. Furthermore, the estimated values of the self-diffusion coefficient are comparable to those in the sulfide-based Li
was reasonably explained by a thermal activation process with the activation energy determined from ionic-conductivity measurements. Furthermore, the estimated values of the self-diffusion coefficient are comparable to those in the sulfide-based Li ion conductor, Li
ion conductor, Li P
P S
S , with 10 times larger ionic conductivity, which clarifies how to understand the Li conduction mechanism in LLTO and Li
, with 10 times larger ionic conductivity, which clarifies how to understand the Li conduction mechanism in LLTO and Li P
P S
S in a unified manner.
in a unified manner.