Phase-field mobility for crystal growth rates in undercooled silicates, SiO and GeO
and GeO liquids
liquids
過冷却中の珪酸塩、SiO , GeO
, GeO 融液中の結晶成長に対する界面易動度
融液中の結晶成長に対する界面易動度
河口 宗道 

 ; 宇埜 正美*
; 宇埜 正美*
Kawaguchi, Munemichi; Uno, Masayoshi*
過冷却ケイ酸塩,SiO ,GeO
,GeO 融液中の11種類の酸化物または混合酸化物の結晶化におけるフェーズフィールド易動度
融液中の11種類の酸化物または混合酸化物の結晶化におけるフェーズフィールド易動度 と結晶成長速度をフェーズフィールドモデル(PFM)を用いて計算し、
と結晶成長速度をフェーズフィールドモデル(PFM)を用いて計算し、 の物質依存性を議論した。実験の結晶成長速度と
の物質依存性を議論した。実験の結晶成長速度と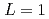 のPFMシミュレーションから得られた結晶成長速度の比は、両対数プロットで結晶成長における固液界面プロセスの
のPFMシミュレーションから得られた結晶成長速度の比は、両対数プロットで結晶成長における固液界面プロセスの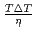 のべき乗に比例した。また
のべき乗に比例した。また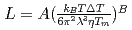 のパラメータ
のパラメータ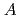 と
と は
は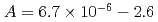 m
m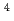 J
J s
s ,
,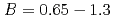 であり、材料に固有の値であることが分かった。決定された
であり、材料に固有の値であることが分かった。決定された を用いたPFMシミュレーションにより、実験の結晶成長速度を定量的に再現することができた。
を用いたPFMシミュレーションにより、実験の結晶成長速度を定量的に再現することができた。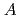 は
は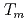 における単位酸素モル質量あたりの陽イオンモル質量の平均の拡散係数と両対数グラフで比例関係にある。
における単位酸素モル質量あたりの陽イオンモル質量の平均の拡散係数と両対数グラフで比例関係にある。 は化合物中の酸素モル質量あたりの陽イオンのモル質量の総和
は化合物中の酸素モル質量あたりの陽イオンのモル質量の総和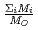 に依存する。
に依存する。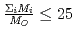 では、陽イオンのモル質量が大きくなるにつれて
では、陽イオンのモル質量が大きくなるにつれて は小さくなる。陽イオンのモル質量は陽イオンの移動の慣性抵抗に比例するため、
は小さくなる。陽イオンのモル質量は陽イオンの移動の慣性抵抗に比例するため、 は陽イオンのモル質量の逆数で減少する。
は陽イオンのモル質量の逆数で減少する。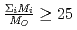 の重い陽イオンのケイ酸塩の結晶化では、
の重い陽イオンのケイ酸塩の結晶化では、 は約
は約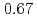 で飽和し、
で飽和し、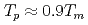 となる。
となる。
Phase-field mobility,  , and crystal growth rates in crystallization of 11 oxides or mixed oxides in undercooled silicates, SiO
, and crystal growth rates in crystallization of 11 oxides or mixed oxides in undercooled silicates, SiO and GeO
and GeO liquids were calculated with a simple phase-field model (PFM), and material dependence of the
liquids were calculated with a simple phase-field model (PFM), and material dependence of the  was discussed. Ratios between experimental crystal growth rates and the PFM simulation with
was discussed. Ratios between experimental crystal growth rates and the PFM simulation with 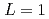 were confirmed to be proportional to a power of
were confirmed to be proportional to a power of 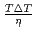 on the solid/liquid interface process during the crystal growth in a log-log plot. We determined that parameters,
on the solid/liquid interface process during the crystal growth in a log-log plot. We determined that parameters, 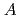 and
and  , of the
, of the 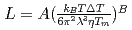 were
were 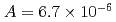 to
to 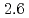 m
m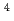 J
J s
s and
and 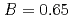 to
to 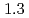 , which were unique for the materials. It was confirmed that our PFM simulation with the determined
, which were unique for the materials. It was confirmed that our PFM simulation with the determined  reproduced quantitively the experimental crystal growth rates. The
reproduced quantitively the experimental crystal growth rates. The 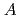 has a proportional relationship with the diffusion coefficient of a cation molar mass average per unit an oxygen molar mass at
has a proportional relationship with the diffusion coefficient of a cation molar mass average per unit an oxygen molar mass at 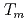 in a log-log graph. The
in a log-log graph. The  depends on the sum of the cation molar mass per the oxygen molar mass,
depends on the sum of the cation molar mass per the oxygen molar mass, 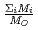 , in a compound. In
, in a compound. In 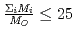 , the
, the  decreases with the cation molar mass increasing. The assumed cause is that the B represents the degree of the temperature dependence of the
decreases with the cation molar mass increasing. The assumed cause is that the B represents the degree of the temperature dependence of the  . Since the cation molar mass is proportional to an inertial resistance of the cation transfer, the
. Since the cation molar mass is proportional to an inertial resistance of the cation transfer, the  decreases with inverse of the cation molar mass. In crystallization of the silicates of heavy cation in
decreases with inverse of the cation molar mass. In crystallization of the silicates of heavy cation in 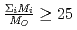 , the
, the  saturates at approximately 0.67, which leads to
saturates at approximately 0.67, which leads to 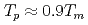 .
.