Comprehensive extraction study using  -dioctylthiodiglycolamic acid; Effect of S donor on metal extraction
-dioctylthiodiglycolamic acid; Effect of S donor on metal extraction
Shimojo, Kojiro 

 ; Fujiwara, Iori*; Saito, Takumi*; Oshima, Tatsuya*
; Fujiwara, Iori*; Saito, Takumi*; Oshima, Tatsuya*
Extraction ability of  -dioctylthiodiglycolamic acid (T-DODGAA), a soft-base sulfur donor ligand with an amide group and a carboxylic acid connected by a thioether chain, for 56 metal ions have been comprehensively investigated and compared with that of N,N-dioctyldiglycolamic acid (DODGAA) with an etheric oxygen atom, a hard-base donor. The p
-dioctylthiodiglycolamic acid (T-DODGAA), a soft-base sulfur donor ligand with an amide group and a carboxylic acid connected by a thioether chain, for 56 metal ions have been comprehensively investigated and compared with that of N,N-dioctyldiglycolamic acid (DODGAA) with an etheric oxygen atom, a hard-base donor. The p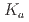 of the thiodiglycolamic acid framework was determined to be 3.71
of the thiodiglycolamic acid framework was determined to be 3.71  0.06 in water (0.1 M LiCl, 25
0.06 in water (0.1 M LiCl, 25 C ) by potentiometric titration, indicating that T-DODGAA is a slightly weaker acid than DODGAA (p
C ) by potentiometric titration, indicating that T-DODGAA is a slightly weaker acid than DODGAA (p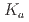 = 3.54
= 3.54  0.03). T-DODGAA can quantitatively extract various metal ions from the 56 metal ions through a proton-exchange reaction. T-DODGAA provided higher extraction performance than DODGAA for Hf(IV), Cr(III), Fe(III), Ni(II), Cu(II), Pd(II), Ag(I), Au(III), Hg(II), Al(III), and Ga(III), especially for soft metal ions. Furthermore, to demonstrate the practical feasibility of T-DODGAA for hydrometallurgy and metal recycling, we performed selective separation tests of rare metal ions such as Sc(III), Ni(II), Co(II), Pd(II), Au(III), In(III), and Ga(II) in metal-mixed extraction systems.
0.03). T-DODGAA can quantitatively extract various metal ions from the 56 metal ions through a proton-exchange reaction. T-DODGAA provided higher extraction performance than DODGAA for Hf(IV), Cr(III), Fe(III), Ni(II), Cu(II), Pd(II), Ag(I), Au(III), Hg(II), Al(III), and Ga(III), especially for soft metal ions. Furthermore, to demonstrate the practical feasibility of T-DODGAA for hydrometallurgy and metal recycling, we performed selective separation tests of rare metal ions such as Sc(III), Ni(II), Co(II), Pd(II), Au(III), In(III), and Ga(II) in metal-mixed extraction systems.