Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
He, X.*; Kagi, Hiroyuki*; Komatsu, Kazuki*; Iizuka, Riko*; Okajima, Hajime*; Hattori, Takanori; Sano, Asami; Machida, Shinichi*; Abe, Jun*; Goto, Hirotada*; et al.
Journal of Molecular Structure, 1310, p.138271_1 - 138271_8, 2024/08
High-pressure responses of the O-D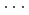 F hydrogen bonds in deuterated magnesium hydroxyfluoride were investigated using neutron powder diffraction and Raman spectroscopy. The Rietveld analysis at ambient conditions revealed a chemical formula of Mg(OD)
F hydrogen bonds in deuterated magnesium hydroxyfluoride were investigated using neutron powder diffraction and Raman spectroscopy. The Rietveld analysis at ambient conditions revealed a chemical formula of Mg(OD)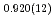 F
F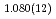 and hydroxyl group/fluorine disorder (OD/F disorder) in the crystal structure, which gave rise to two hydrogen-bonding configurations. The Rietveld analysis showed the hydrogen-bonding geometries remains up to 9.8 GPa, indicating no pressure-induced strengthening of hydrogen bonds. The Raman spectra at ambient conditions showed three hydroxyl stretching bands at 2613, 2694, and 2718 cm
and hydroxyl group/fluorine disorder (OD/F disorder) in the crystal structure, which gave rise to two hydrogen-bonding configurations. The Rietveld analysis showed the hydrogen-bonding geometries remains up to 9.8 GPa, indicating no pressure-induced strengthening of hydrogen bonds. The Raman spectra at ambient conditions showed three hydroxyl stretching bands at 2613, 2694, and 2718 cm . The high frequencies of the O-D stretching modes indicated that the hydroxyls should be involved in weak or none hydrogen-bonding interactions. Up to 20.2 GPa, the mode initially centered at 2694 cm
. The high frequencies of the O-D stretching modes indicated that the hydroxyls should be involved in weak or none hydrogen-bonding interactions. Up to 20.2 GPa, the mode initially centered at 2694 cm displayed a pressure-induced blue shift, revealing no strengthening of hydrogen bonds under compression. We discuss the existence of hydrogen bonds and the causes of the blue-shifting hydroxyls at ambient and at high pressures.
displayed a pressure-induced blue shift, revealing no strengthening of hydrogen bonds under compression. We discuss the existence of hydrogen bonds and the causes of the blue-shifting hydroxyls at ambient and at high pressures.