Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 10
10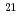 W/cm
W/cm
Skobelev, I. Yu.*; Ryazantsev, S. N.*; Kulikov, R. K.*; Sedov, M. V.*; Filippov, E. D.*; Pikuz, S. A.*; Asai, Takafumi*; Kanasaki, Masato*; Yamauchi, Tomoya*; Jinno, Satoshi; et al.
Photonics (Internet), 10(11), p.1250_1 - 1250_11, 2023/11
Times Cited Count:0 Percentile:0.00(Optics)It is challenging to clearly distinguish the impacts of the optical field and collisional ionization in the evolution of the charge state of a plasma produced when matter interacts with high-intensity laser pulses. In this work, time-dependent calculations of plasma kinetics are used to show that it is possible only when low-density gaseous targets with sufficiently small clusters are used. In the case of Ar plasma, the upper limit of the cluster radius was estimated to be 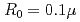 m.
m.
 isomers in
isomers in  Cf
CfOrlandi, R.; Makii, Hiroyuki; Nishio, Katsuhisa; Hirose, Kentaro; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Ito, Yuta; Suzaki, Fumi; Nagame, Yuichiro*; et al.
Physical Review C, 106(6), p.064301_1 - 064301_11, 2022/12
Times Cited Count:1 Percentile:28.09(Physics, Nuclear)
Chiera, N. M.*; Sato, Tetsuya; Eichler, R.*; Tomitsuka, Tomohiro; Asai, Masato; Adachi, Sadia*; Dressler, R.*; Hirose, Kentaro; Inoue, Hiroki*; Ito, Yuta; et al.
Angewandte Chemie; International Edition, 60(33), p.17871 - 17874, 2021/08
Times Cited Count:3 Percentile:21.36(Chemistry, Multidisciplinary)The formation and the chemical characterization of single atoms of dubnium (Db, element 105), in the form of its volatile oxychloride, was investigated using the on-line gas phase chromatography technique, in the temperature range 350 - 600  C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl
C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl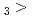 TaOCl
TaOCl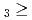 DbOCl
DbOCl . From the obtained experimental results, thermochemical data for DbOCl
. From the obtained experimental results, thermochemical data for DbOCl were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
 and 3
and 3 two-proton states in
two-proton states in 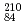 Po
Po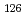 populated by two-proton transfer
populated by two-proton transferDupont, E.*; Astier, A.*; Petrache, C. M.*; Lv, B. F.*; Deloncle, I.*; Kiener, J.*; Orlandi, R.; Makii, Hiroyuki; Nishio, Katsuhisa; Hirose, Kentaro; et al.
Physical Review C, 101(1), p.014309_1 - 014309_6, 2020/01
Times Cited Count:2 Percentile:24.58(Physics, Nuclear) decay of
decay of 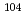 Te with a novel recoil-decay scintillation detector
Te with a novel recoil-decay scintillation detectorXiao, Y.*; Go, S.*; Grzywacz, R.*; Orlandi, R.; Andreyev, A. N.; Asai, Masato; Bentley, M. A.*; de Angelis, G.*; Gross, C. J.*; Hausladen, P.*; et al.
Physical Review C, 100(3), p.034315_1 - 034315_8, 2019/09
Times Cited Count:16 Percentile:83.79(Physics, Nuclear)Nishio, Katsuhisa; Hirose, Kentaro; Vermeulen, M. J.; Makii, Hiroyuki; Orlandi, R.; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Sato, Tetsuya; Nagame, Yuichiro; et al.
EPJ Web of Conferences, 163, p.00041_1 - 00041_6, 2017/11
Times Cited Count:1 Percentile:60.69(Nuclear Science & Technology)Sawaki, Kenta*; Asai, Shiho; Watanabe, Kazuo; Sugo, Takanobu*; Saito, Kyoichi*
Maku, 33(1), p.32 - 38, 2008/01
TOPO, tri-n-octylphosphine oxide, as a neutral extractant was impregnated to the hydrophobic group of the polymer chain grafted onto a porous hollow-fiber membrane for an efficient metal-ion collection. A diol group,i.e., adjacent hydroxyl group was introduced into poly-glycidyl methacrylate(GMA) chain grafted onto the porous hollow-fiber membrane via the epoxy-ring-opening reaction with water, followed by a reaction of the remaining epoxy group with octadecanethiol (C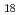 H
H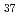 SH) to form an octadecanethiol group. The diol group reduced the amount of TOPO coagulatedon the graft chain, whereas the interaction of the octadecanethiol group of the graft chain with the hydrophobic part of TOPO enabled the impregnation of TOPO to the graft chain. A 1 mmol-Bi/L bismuth chloride solution was forced to permeate through the pores of the TOPO-impregnated porous hollow-fiber membrane with a density of TOPO impregnated of 1.3 mol/kg of the GMA-grafted porous hollow-fiber membrane and the pure water flux of 0.7 m/h at 0.1 MPa and 298 K. The equilibrium binding capacity of the membrane for bismuth ions was 0.22 mol/kg of the TOPO-impregnated porous hollow-fiber membrane, which was equivalent to a binding ratio of bismuth ion to impregnated TOPO of 1.0.
SH) to form an octadecanethiol group. The diol group reduced the amount of TOPO coagulatedon the graft chain, whereas the interaction of the octadecanethiol group of the graft chain with the hydrophobic part of TOPO enabled the impregnation of TOPO to the graft chain. A 1 mmol-Bi/L bismuth chloride solution was forced to permeate through the pores of the TOPO-impregnated porous hollow-fiber membrane with a density of TOPO impregnated of 1.3 mol/kg of the GMA-grafted porous hollow-fiber membrane and the pure water flux of 0.7 m/h at 0.1 MPa and 298 K. The equilibrium binding capacity of the membrane for bismuth ions was 0.22 mol/kg of the TOPO-impregnated porous hollow-fiber membrane, which was equivalent to a binding ratio of bismuth ion to impregnated TOPO of 1.0.
Sawaki, Kenta*; Domon, Sayaka*; Asai, Shiho; Watanabe, Kazuo; Sugo, Takanobu*; Saito, Kyoichi*
Maku, 32(2), p.109 - 115, 2007/03
no abstracts in English
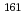 Sm and
Sm and 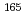 Gd
GdIchikawa, Shinichi; Tsukada, Kazuaki; Nishinaka, Ichiro; Iimura, Hideki; ; Nagame, Yuichiro; Asai, Masato*; Kojima, Yasuaki*; ; Shibata, M.*; et al.
Physical Review C, 58(2), p.1329 - 1332, 1998/08
Times Cited Count:13 Percentile:60.07(Physics, Nuclear)no abstracts in English
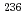 Am
AmTsukada, Kazuaki; Ichikawa, Shinichi; Hatsukawa, Yuichi; Nishinaka, Ichiro; ; Nagame, Yuichiro; Oura, Yasutsugu*; ; Sueki, Keisuke*; ; et al.
Physical Review C, 57(4), p.2057 - 2060, 1998/04
Times Cited Count:15 Percentile:63.92(Physics, Nuclear)no abstracts in English
Hirose, Kentaro; Nishio, Katsuhisa; Nishinaka, Ichiro; Makii, Hiroyuki; Ikezoe, Hiroshi*; Orlandi, R.; L guillon, R.; Tsukada, Kazuaki; Asai, Masato; Nagame, Yuichiro; et al.
guillon, R.; Tsukada, Kazuaki; Asai, Masato; Nagame, Yuichiro; et al.
no journal, ,
no abstracts in English
Hirose, Kentaro; Nishio, Katsuhisa; L guillon, R.; Makii, Hiroyuki; Nishinaka, Ichiro; Orlandi, R.; Smallcombe, J.; Ishii, Tetsuro; Tsukada, Kazuaki; Asai, Masato; et al.
guillon, R.; Makii, Hiroyuki; Nishinaka, Ichiro; Orlandi, R.; Smallcombe, J.; Ishii, Tetsuro; Tsukada, Kazuaki; Asai, Masato; et al.
no journal, ,
Sato, Tetsuya; Kaneya, Yusuke*; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina*; Osa, Akihiko; Makii, Hiroyuki; Nishio, Katsuhisa; Hirose, Kentaro; et al.
no journal, ,
The ground state electronic configuration of lawrencium (Lr, Z =103) is predicted to be [Rn]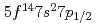 , which is different from that of the lanthanide homolog Lu [Xe]
, which is different from that of the lanthanide homolog Lu [Xe]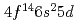 due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of
due to strong relativistic effects. According to semi-empirical considerations, volatility of Lr is expected to be higher than that of Lu. We have investigated adsorption behavior of  Lr, which was produced in the reaction of
Lr, which was produced in the reaction of  Cf(
Cf(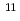 B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of
B, 4n), on a tantalum (Ta) metal surface using a surface ion-source installed into the isotope separator on-line (ISOL) at the JAEA tandem accelerator facility. The observed adsorption behavior of  Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.
Lr was similar to those of Tb and Lu which have relatively higher adsorption enthalpy on Ta surface. It implies that Lr would have low volatility like such as Lu and Tb.
 -ray spectroscopy of the heaviest nuclei at the JAEA-Tokai Tandem laboratory, using
-ray spectroscopy of the heaviest nuclei at the JAEA-Tokai Tandem laboratory, using  Cf and
Cf and 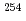 Es targets
Es targetsOrlandi, R.; Makii, Hiroyuki; Nishio, Katsuhisa; Hirose, Kentaro; Tsukada, Kazuaki; Asai, Masato; Nagame, Yuichiro; Sato, Tetsuya; Toyoshima, Atsushi; Vermeulen, M. J.; et al.
no journal, ,
L guillon, R.; Nishio, Katsuhisa; Hirose, Kentaro; Orlandi, R.; Makii, Hiroyuki; Nishinaka, Ichiro; Ishii, Tetsuro; Tsukada, Kazuaki; Asai, Masato; Chiba, Satoshi*; et al.
guillon, R.; Nishio, Katsuhisa; Hirose, Kentaro; Orlandi, R.; Makii, Hiroyuki; Nishinaka, Ichiro; Ishii, Tetsuro; Tsukada, Kazuaki; Asai, Masato; Chiba, Satoshi*; et al.
no journal, ,
Hirose, Kentaro; Nishio, Katsuhisa; Ikezoe, Hiroshi; Mitsuoka, Shinichi; Nishinaka, Ichiro; Makii, Hiroyuki; Wakabayashi, Yasuo*; Tsukada, Kazuaki; Asai, Masato; Nagame, Yuichiro; et al.
no journal, ,
Sawaki, Kenta*; Asai, Shiho; Domon, Sayaka*; Saito, Kyoichi*; Sugo, Takanobu*
no journal, ,
no abstracts in English
Kaneya, Yusuke*; Asai, Masato; Sato, Tetsuya; Tomitsuka, Tomohiro; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina; Makii, Hiroyuki; Hirose, Kentaro; Osa, Akihiko; et al.
no journal, ,
To study the influence of the valence 7p electronic orbital on chemical properties of lawrencium, a measurement of the adsorption enthalpy of lawrencium was carried out. A new method using a surface ionization technique coupled to an on-line isotope separator was developed, which enabled one to measure temperature dependence of lawrencium surface adsorption on a metallic tantalum surface at high temperature up to 2800 K. The temperature dependences of adsorption of lawrencium as well as various lanthanide elements were investigated with this method, and the adsorption enthalpy of lawrencium was successfully extracted.
electronic orbital on chemical properties of lawrencium, a measurement of the adsorption enthalpy of lawrencium was carried out. A new method using a surface ionization technique coupled to an on-line isotope separator was developed, which enabled one to measure temperature dependence of lawrencium surface adsorption on a metallic tantalum surface at high temperature up to 2800 K. The temperature dependences of adsorption of lawrencium as well as various lanthanide elements were investigated with this method, and the adsorption enthalpy of lawrencium was successfully extracted.
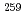 Lr
LrAsai, Masato; Kamada, Hiroki*; Shigekawa, Yudai*; Tsukada, Kazuaki; Sato, Tetsuya; Toyoshima, Atsushi; Mitsukai, Akina; Nagame, Yuichiro; Nishio, Katsuhisa; Hirose, Kentaro; et al.
no journal, ,
To clarify the fission mechanism of mass-symmetric spontaneous fissions observed around the neutron-rich Fm region, we have measured kinetic energies and mass distribution of spontaneous-fission fragments in this nuclear region precisely. The experiment was performed at the JAEA tandem accelerator facility using an on-line isotope separator (ISOL). The mass-separated 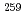 Lr nuclei were implanted into thin foil, and two fragments of their spontaneous fission were coincidently measured with two Si detectors. The spontaneous fission of
Lr nuclei were implanted into thin foil, and two fragments of their spontaneous fission were coincidently measured with two Si detectors. The spontaneous fission of 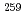 Lr was found to show a narrow symmetric mass distribution. On the other hand, their total kinetic energy TKE was found to be low, which is apparently different from the high-TKE symmetric fissions observed in the Fm isotopes. This indicates that the fission mechanisms of these two symmetric fissions are different.
Lr was found to show a narrow symmetric mass distribution. On the other hand, their total kinetic energy TKE was found to be low, which is apparently different from the high-TKE symmetric fissions observed in the Fm isotopes. This indicates that the fission mechanisms of these two symmetric fissions are different.
Kaneya, Yusuke*; Tomitsuka, Tomohiro; Sato, Tetsuya; Asai, Masato; Tsukada, Kazuaki; Toyoshima, Atsushi; Mitsukai, Akina; Makii, Hiroyuki; Hirose, Kentaro; Osa, Akihiko; et al.
no journal, ,