Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
; ; ;
JNC TJ8400 2001-024, 71 Pages, 2001/03
Crystallization procedure is considered to have an advantage in recovering rather pure uranium from contaminated uranium solution and to be applicable for a new reprocessing process. It is considered necessary to collect data for Pu crystallization for design of the process with crystallization procedure. Last year the test for Pu(IV) nitrate erystallization was performed and it was confirmed that Pu crystallization is not observed under supposed crystallization condition if Pu valence is adjusted to 4. In this study, two type beaker tests were performed, (1)Pu(VI) nitrate crystallization test to confirm a behavior of Pu(VI) nitrate under crystallization condition. (2)U-Pu(VI) nitrate crystallization test to confirm a U-Pu(VI) co-crystallization phenomena. These tests were performed in AEA Technology Harwell Laboratory and the results were examined by Mitsubishi Materials Corporation. Test results were as follows. (1)Pu(VI) crystallization test. (a)Pu(VI) nitrate solution of 200, 100 and 50 gPu/L with HNO 6M were cooled down up to -60
6M were cooled down up to -60 C to confirm Pu(VI) nitrate crystallization or freezing of the solution. (b)Crystal of H
C to confirm Pu(VI) nitrate crystallization or freezing of the solution. (b)Crystal of H O and HNO
O and HNO
 3H
3H O were observed but Pu(VI) nitrate crystallization was not observed. (c)We can estimate that Pu(VI) nitrate crystallization will not occurred in the reprocessing process with crystallization procedure. (2) U-Pu(VI) nitrate crystallization test. (a)U-Pu(VI) mixed nitrate solution is cooled to 10
O were observed but Pu(VI) nitrate crystallization was not observed. (c)We can estimate that Pu(VI) nitrate crystallization will not occurred in the reprocessing process with crystallization procedure. (2) U-Pu(VI) nitrate crystallization test. (a)U-Pu(VI) mixed nitrate solution is cooled to 10 C and 0
C and 0 C. (b)U-Pu(VI) co-crystallization was confirmed by orange colored crystal in both cooling temperatures. (c) It is considered that Pu(VI) nitrate crystal is co-crystallized with uranyl nitrate crystal by the following reasons.
C. (b)U-Pu(VI) co-crystallization was confirmed by orange colored crystal in both cooling temperatures. (c) It is considered that Pu(VI) nitrate crystal is co-crystallized with uranyl nitrate crystal by the following reasons. 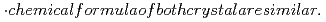 cdot$ crystal form is same and lattice paramters are very near. (d)U+Pu(VI) crystallization data is very near with uranyl nitrate crystallization data if Pu(VI) nitrate is considered to be crystallized ...
cdot$ crystal form is same and lattice paramters are very near. (d)U+Pu(VI) crystallization data is very near with uranyl nitrate crystallization data if Pu(VI) nitrate is considered to be crystallized ...
; ; ;
JNC TJ8400 2000-061, 92 Pages, 2000/03
Crystallization procedure is considered to have an advantage in recovering rather pure uranium from contaminated uranium solution and to be applicable for a new reprocessing process. It was confirmed until last year that the reprocessing process with crystallization procedure has a sufficient advantage. But the data for Pu crystallization is very rare. although it is necessary for design of the process with crystallization procedure. In this study, a beaker scale plutonium test was performed in AEA Technology Harwell Laboratory to confirm a behavior of Pu (IV) nitrate under crystallization condition. The results were examined by Mitsubishi Materials Corporation. Test item was a measurement of temperature in case of Pu (IV) nitrate crystallization or freezing of the solution in the following six parameters. (Pu(g/L):200, 100, 50, HNO (m):6, Pu valence:4). (Pu(g/L):200, 100, 50, HNO
(m):6, Pu valence:4). (Pu(g/L):200, 100, 50, HNO (m):4, Pu valence:4). Test results were as follows. (1)Pu(IV) nitrate crystallization was not observed even in the case 200g Pu/L and HNO
(m):4, Pu valence:4). Test results were as follows. (1)Pu(IV) nitrate crystallization was not observed even in the case 200g Pu/L and HNO 6M and 4M which were considered to the best condition but crystal of H
6M and 4M which were considered to the best condition but crystal of H O and HNO
O and HNO
 3H
3H O were observed. (2)Similar results were obtained for the other parameter with lower Pu concentration. (3)We can estimate that Pu(IV) nitrate crystallization will not occurred in the reprocessing process with crystallization procedure. (4)The solubility data of Pu(NO
O were observed. (2)Similar results were obtained for the other parameter with lower Pu concentration. (3)We can estimate that Pu(IV) nitrate crystallization will not occurred in the reprocessing process with crystallization procedure. (4)The solubility data of Pu(NO )
) - HNO
- HNO -H
-H O system was obtained.
O system was obtained.
Sugikawa, Susumu; ; Takeshita, Isao; ; ; ; P.Bretault*; R.Vialard*; M.Lecomte*
Proc. of 5th Int. Nucl. Conf. on Recycling, Conditioning and Disposal (RECOD '98), 3, p.931 - 938, 1998/00
no abstracts in English
Maeda, Mitsuru; Fujine, Sachio; ;
Transactions of the American Nuclear Society, 66, p.78 - 80, 1992/11
no abstracts in English
Yokobori, Shinichi*; Haruyama, Junichi*; Yano, Hajime*; Narumi, Issei; Mita, Hajime*; Takahashi, Junichi*
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Haruyama, Junichi*; Yano, Hajime*; Narumi, Issei; Mita, Hajime*; Takahashi, Junichi*
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Yang, Y.*; Kawaguchi, Yuko*; Sugino, Tomohiro*; Takahashi, Yuta*; Narumi, Issei; Takahashi, Yuichi*; Hayashi, Nobuhiro*; Yoshimura, Yoshitaka*; Tabata, Makoto*; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Yang, Y.*; Sugino, Tomohiro*; Kawaguchi, Yuko*; Takahashi, Yuta*; Narumi, Issei; Hashimoto, Hirofumi*; Hayashi, Nobuhiro*; Imai, Eiichi*; Kawai, Hideyuki*; et al.
no journal, ,
Yokobori, Shinichi*; Kobayashi, Kensei*; Mita, Hajime*; Yabuta, Hikaru*; Nakagawa, Kazumichi*; Narumi, Issei; Hayashi, Nobuhiro*; Tomita, Kaori*; Kawaguchi, Yuko*; Shimizu, Yasuyuki*; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Kawaguchi, Yuko*; Yang, Y.*; Kawashiri, Narutoshi*; Shiraishi, Keisuke*; Shimizu, Yasuyuki*; Takahashi, Yuta*; Sugino, Tomohiro*; Narumi, Issei; Sato, Katsuya; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Kawaguchi, Yuko*; Yang, Y.*; Kawashiri, Narutoshi*; Shiraishi, Keisuke*; Shimizu, Yasuyuki*; Takahashi, Yuta*; Sugino, Tomohiro*; Narumi, Issei; Sato, Katsuya; et al.
no journal, ,
no abstracts in English
Yamagishi, Akihiko*; Yokobori, Shinichi*; Hashimoto, Hirofumi*; Yano, Hajime*; Imai, Eiichi*; Okudaira, Kyoko*; Kawai, Hideyuki*; Kobayashi, Kensei*; Tabata, Makoto*; Nakagawa, Kazumichi*; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Hashimoto, Hirofumi*; Hayashi, Nobuhiro*; Imai, Eiichi*; Kawai, Hideyuki*; Kobayashi, Kensei*; Mita, Hajime*; Nakagawa, Kazumichi*; Narumi, Issei; Okudaira, Kyoko*; et al.
no journal, ,
Yokobori, Shinichi*; Kawaguchi, Yuko*; Harada, Miyu*; Murano, Yuka*; Tomita, Kaori*; Hayashi, Nobuhiro*; Tabata, Makoto*; Kawai, Hideyuki*; Okudaira, Kyoko*; Imai, Eiichi*; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Kawaguchi, Yuko*; Shimizu, Yasuyuki*; Kawashiri, Narutoshi*; Shiraishi, Keisuke*; Sugino, Tomohiro*; Takahashi, Yuta*; Yang, Y.*; Tanigawa, Yoshiaki*; Hashimoto, Hirofumi*; et al.
no journal, ,
no abstracts in English
Yano, Yoko*; Tanida, Hajime
no journal, ,
This study group made use of X-ray reflection (XR), grazing-incident X-ray diffraction (GIXD), and X-ray absorption fine structure (XAFS) methods using the high-brilliance synchrotron radiation of SPring-8 to study a soft interface and a soft interface firm in situ.
Yokobori, Shinichi*; Yang, Y.*; Sugino, Tomohiro*; Kawaguchi, Yuko*; Itahashi, Shiho*; Fujisaki, Kenta*; Fushimi, Hidehiko*; Hasegawa, Sunao*; Hashimoto, Hirofumi*; Hayashi, Nobuhiro*; et al.
no journal, ,
Yokobori, Shinichi*; Yang, Y.*; Fujisaki, Kenta*; Kawaguchi, Yuko*; Kobayashi, Kensei*; Hashimoto, Hirofumi*; Kawai, Hideyuki*; Mita, Hajime*; Narumi, Issei; Okudaira, Kyoko*; et al.
no journal, ,
no abstracts in English
Yokobori, Shinichi*; Yang, Y.*; Sugino, Tomohiro*; Kawaguchi, Yuko*; Fushimi, Hidehiko*; Narumi, Issei; Hashimoto, Hirofumi*; Hayashi, Nobuhiro*; Kawai, Hideyuki*; Kobayashi, Kensei*; et al.
no journal, ,