Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakamura, Satoshi; Ishii, Sho*; Kato, Hitoshi*; Ban, Yasutoshi; Hiruta, Kenta; Yoshida, Takuya; Uehara, Hiroyuki; Obata, Hiroki; Kimura, Yasuhiko; Takano, Masahide
Journal of Nuclear Science and Technology, 62(1), p.56 - 64, 2025/01
Times Cited Count:1 Percentile:43.12(Nuclear Science & Technology)A dissolution method for analyzing the elemental composition of fuel debris using the sodium peroxide (Na O
O ) fusion technique has been developed. Herein, two different types of simulated debris materials (such as solid solution of (Zr,RE)O
) fusion technique has been developed. Herein, two different types of simulated debris materials (such as solid solution of (Zr,RE)O and molten core-concrete interaction products (MCCI)) were taken. At various temperatures, these debris materials were subsequently fused with Na
and molten core-concrete interaction products (MCCI)) were taken. At various temperatures, these debris materials were subsequently fused with Na O
O in crucibles, which are made of different materials, such as Ni, Al
in crucibles, which are made of different materials, such as Ni, Al O
O , Fe, and Zr. Then, the fused samples are dissolved in nitric acid. Furthermore, the effects of the experimental conditions on the elemental composition analysis were evaluated using inductively coupled plasma-atomic emission spectroscopy (ICP-AES), which suggested the use of a Ni crucible at 923 K as an optimum testing condition. The optimum testing condition was then applied to the demonstration tests with Three Mile Island unit-2 (TMI-2) debris in a shielded concrete cell, thereby achieving complete dissolution of the debris. The elemental composition of TMI-2 debris revealed by the proposed dissolution method has good reproducibility and has an insignificant contradiction in the mass balance of the sample. Therefore, this newly developed reproducible dissolution method can be effectively utilized in practical applications by dissolving fuel debris and estimating its elemental composition.
, Fe, and Zr. Then, the fused samples are dissolved in nitric acid. Furthermore, the effects of the experimental conditions on the elemental composition analysis were evaluated using inductively coupled plasma-atomic emission spectroscopy (ICP-AES), which suggested the use of a Ni crucible at 923 K as an optimum testing condition. The optimum testing condition was then applied to the demonstration tests with Three Mile Island unit-2 (TMI-2) debris in a shielded concrete cell, thereby achieving complete dissolution of the debris. The elemental composition of TMI-2 debris revealed by the proposed dissolution method has good reproducibility and has an insignificant contradiction in the mass balance of the sample. Therefore, this newly developed reproducible dissolution method can be effectively utilized in practical applications by dissolving fuel debris and estimating its elemental composition.
Tanimura, Yoshihiko; Yoshitomi, Hiroshi; Nishino, Sho; Tsuji, Tomoya; Fukami, Tomoyo; Shinozuka, Tomoki; Oishi, Kohei; Ishii, Masato; Takamiya, Kei; Onuki, Takaya; et al.
Radiation Measurements, 176, p.107196_1 - 107196_6, 2024/08
Times Cited Count:1 Percentile:43.12(Nuclear Science & Technology)The ICRU has proposed to change the definitions of the operational quantities used for the area and individual monitoring for external exposure in the ICRU Report 95. As introducing the new operational quantities into the radiation monitoring may affect the dose assessment results using the present personal dosimeters, it is necessary to characterize the energy spectrum in the workplace and the energy dependency of the dosimeters to be used. In this work the photon spectra were measured using a NaI(Tl) scintillation detector or a LaBr (Ce) scintillation detector at the workplaces in the Japanese Research Reactor No.3 (JRR-3) and the Japan Proton Accelerator Research Complex (J-PARC) at Japan Atomic Energy Agency (JAEA). Then the present and new operational quantities were evaluated using the above mention spectra at the workplaces and compared each other.
(Ce) scintillation detector at the workplaces in the Japanese Research Reactor No.3 (JRR-3) and the Japan Proton Accelerator Research Complex (J-PARC) at Japan Atomic Energy Agency (JAEA). Then the present and new operational quantities were evaluated using the above mention spectra at the workplaces and compared each other.
Ohshima, Hiroyuki; Morishita, Masaki*; Aizawa, Kosuke; Ando, Masanori; Ashida, Takashi; Chikazawa, Yoshitaka; Doda, Norihiro; Enuma, Yasuhiro; Ezure, Toshiki; Fukano, Yoshitaka; et al.
Sodium-cooled Fast Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.3, 631 Pages, 2022/07
This book is a collection of the past experience of design, construction, and operation of two reactors, the latest knowledge and technology for SFR designs, and the future prospects of SFR development in Japan. It is intended to provide the perspective and the relevant knowledge to enable readers to become more familiar with SFR technology.
Kusano, Shogo*; Matsumura, Daiju; Ishii, Kenji*; Tanaka, Hirohisa*; Mizuki, Junichiro*
Nanomaterials (Internet), 9(4), p.642_1 - 642_14, 2019/04
Times Cited Count:10 Percentile:41.64(Chemistry, Multidisciplinary) -based simulated fuel containing CsI
-based simulated fuel containing CsITakamatsu, Yuki*; Ishii, Hiroto*; Oishi, Yuji*; Muta, Hiroaki*; Yamanaka, Shinsuke*; Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Kurosaki, Ken*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 17(3/4), p.106 - 110, 2018/12
In order to establish the synthesis method of simulated fuel contacting Cesium (Cs) which is required for the evaluation of physical/chemical characteristics in fuel and release behavior of Cs, sintering tests of the cerium dioxide (CeO ) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO
) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.
pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.
 ,
, -di(2-ethylhexyl)octanamide from nitric acid medium
-di(2-ethylhexyl)octanamide from nitric acid mediumTsutsui, Nao; Ban, Yasutoshi; Sagawa, Hiroshi; Ishii, Sho; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 35(6), p.439 - 449, 2017/08
Times Cited Count:7 Percentile:23.53(Chemistry, Multidisciplinary)Solvent extraction of uranium from a nitric acid medium was performed with  ,
, -di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as
-di(2-ethylhexyl)octanamide (DEHOA) by a single-stage batch method, and the distribution ratio equation of U(VI) was derived as 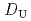 = 1.1
= 1.1![$$[rm NO^{-}_{3}]^{1.6}_{rm aq}[{rm DEHOA}]^{2}_{rm org}$$](/search/images/ERNVY2TNEBHE4XT1FV4V44ZTPVOV24ZRFY1H0X11LRZG0IDBOF4VW402OJWSARCFJBHUC5K3LZ3TE5K5PNOHE1JAN3ZGO5JE.gif) . Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation
. Furthermore, the nitric acid distribution was also evaluated, and the distribution ratio equation 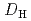 = 0.12
= 0.12![$$[rm H^{+}]^{0.76}_{rm aq}[{rm DEHOA_{rm Free}}]_{rm H}$$](/search/images/ERNVY2TNEBEF24ZLPVOV24ZQFY1TM5K5PNOHE1JAMFYX0W11LRZG0ICEIVEE4QK5PNOHE1JAIZZGKZL3PVOV4402OJWSASD3EQ.gif) was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm
was obtained. Batch experiments to evaluate the time dependence of U(VI) extraction and the U(VI) loading capacity of DEHOA were also performed. It was revealed that U(VI) extraction by DEHOA reached an equilibrium state within a few minutes, and the loading capacity was 0.71 mol/dm (M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
(M) when the concentrations of DEHOA and nitric acid were 1.5 and 3.0 M, respectively.
Kawasaki, Masatsugu; Nakajima, Junya; Yoshida, Keisuke; Kato, Saori; Nishino, Sho; Nozaki, Teo; Nakagawa, Masahiro; Tsunoda, Junichi; Sugaya, Yuki; Hasegawa, Rie; et al.
JAEA-Data/Code 2017-004, 57 Pages, 2017/03
In emergency situation of nuclear facilities, we need to estimate the radiation dose due to radiation and radioactivity to grasp the influence range of the accident in the early stage. Therefore, we prepare the case studies of dose assessment for public exposure dose and personal exposure dose and contribute them to emergency procedures. This document covers about accidents of nuclear facilities in Nuclear Science Research Institute and past accident of nuclear power plant, and it can be used for inheritance of techniques of emergency dose assessment.
 ,
, -di(2-ethylhexyl)octanamide toward uranium from nitric acid
-di(2-ethylhexyl)octanamide toward uranium from nitric acidTsutsui, Nao; Ban, Yasutoshi; Sagawa, Hiroshi; Ishii, Sho; Matsumura, Tatsuro
Proceedings of 5th International Conference on Asian Nuclear Prospects 2016 (ANUP 2016) (CD-ROM), 2 Pages, 2016/10
Extraction properties of  ,
, -di(2-ethylhexyl)octanamide (DEHOA) toward uranium from nitric acid were studied using single-stage batch method. The results revealed that DEHOA effectively extracted uranium when the nitrate ion concentration was 2.0-3.0 mol/dm
-di(2-ethylhexyl)octanamide (DEHOA) toward uranium from nitric acid were studied using single-stage batch method. The results revealed that DEHOA effectively extracted uranium when the nitrate ion concentration was 2.0-3.0 mol/dm .
.
Sekimoto, Hitoshi*; Kawachi, Naoki; Honda, Shuzo*; Yamaguchi, Yoshie*; Kato, Shota*; Yoneyama, Kaori*; Fujimaki, Shu; Suzui, Nobuo; Ishii, Satomi; Watanabe, Satoshi; et al.
JAEA-Review 2007-060, JAEA Takasaki Annual Report 2006, P. 124, 2008/03
no abstracts in English
Sekimoto, Hitoshi; Honda, Shuzo*; Kato, Shota*; Ochiai, Yukiko*; Yoneyama, Kaori*; Yoneyama, Koichi*; Takeuchi, Yasutomo*; Kawachi, Naoki; Fujimaki, Shu; Suzui, Nobuo; et al.
JAEA-Review 2006-042, JAEA Takasaki Annual Report 2005, P. 125, 2007/02
 -radiolysis of MA separation agent of HONTA on extraction performance
-radiolysis of MA separation agent of HONTA on extraction performanceToigawa, Tomohiro; Suzuki, Hideya; Ban, Yasutoshi; Ishii, Sho*; Matsumura, Tatsuro
no journal, ,
To evaluate the radiolytic stability of a novel tetradentate extractant of N, N, N', N', N", N"-hexaoctyl-nitrilotriacetamide (HONTA), batch extractions of lanthanide (Ln) elements were performed by using  -irradiated HONTA extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no difference between Ln could be observed. These indicate that the degradation products of HONTA did not act as Ln extractants, and the decay of Ln extraction were caused by degradation of HONTA.
-irradiated HONTA extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no difference between Ln could be observed. These indicate that the degradation products of HONTA did not act as Ln extractants, and the decay of Ln extraction were caused by degradation of HONTA.
 -based simulated fuel containing CsI
-based simulated fuel containing CsITakamatsu, Yuki*; Kurosaki, Ken*; Ishii, Hiroto*; Oishi, Yuji*; Muta, Hiroaki*; Yamanaka, Shinsuke*; Nakajima, Kunihisa; Suzuki, Eriko; Miwa, Shuhei; Osaka, Masahiko
no journal, ,
We performed spark plasma sintering tests of the cerium dioxide (CeO )-based simulated fuel containing cesium iodide (CsI), in order to establish a synthesis technology of simulated fuel including volatile fission product (FP) such as cesium (Cs) and iodine (I) for a study on the FP release behavior. We could successfully fabricated the simulated fuel containing CsI by the spark plasma sintering at lower temperature and in shorter time. It has homogeneous distribution of CsI compound in the sintered pellet.
)-based simulated fuel containing cesium iodide (CsI), in order to establish a synthesis technology of simulated fuel including volatile fission product (FP) such as cesium (Cs) and iodine (I) for a study on the FP release behavior. We could successfully fabricated the simulated fuel containing CsI by the spark plasma sintering at lower temperature and in shorter time. It has homogeneous distribution of CsI compound in the sintered pellet.
 -radiolysis of a minor actinide extractnt on lanthanides extraction
-radiolysis of a minor actinide extractnt on lanthanides extractionToigawa, Tomohiro; Suzuki, Hideya; Ishii, Sho*; Tsutsui, Nao; Ban, Yasutoshi; Matsumura, Tatsuro
no journal, ,
To evaluate the radiolytic stability of an extractant agents for minor actinides such as N, N, N', N', N", N"-hexaoctyl-nitrilotriacetamide (HONTA), batch extractions of lanthanide (Ln) elements were performed by using  -irradiated extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no initial decrease could be observed.
-irradiated extraction solvents. The distribution ratios decreased exponentially with increasing the absorbed dose, and no initial decrease could be observed.
Matsumura, Tatsuro; Ban, Yasutoshi; Suzuki, Hideya; Tsubata, Yasuhiro; Hotoku, Shinobu; Tsutsui, Nao; Suzuki, Asuka; Toigawa, Tomohiro; Kurosawa, Tatsuya*; Shibata, Mitsunobu*; et al.
no journal, ,
PUREX process was established for industrial scale reprocessing plant. TRUEX and the 4 group separation were developed for partitioning of minor actinides from high level liquid waste from reprocessing process, and demonstrated by the continuous extraction test using genuine high level liquid waste. Although the extractants for reprocessing and MA separation processes, such as tri-n-butyl phosphate (TBP), n-octyl(phenyl)-N, N-diisobutylcarbamoylmethylphosphine oxide (CMPO) and diisodecylphosphoric acid (DIDPA), have excellent performance for recovery of U, Pu or MA, the molecules contain phosphorus which could be cause for the secondary waste from the solvent extraction processes. To minimize the radioactive waste from nuclear fuel cycle, we have conducted research and development of the new reprocessing and MA separation processes using innovative extractants in accord with CHON principle.
Tsutsui, Nao; Ban, Yasutoshi; Ishii, Sho*; Ito, Sayumi*; Suzuki, Hideya; Matsumura, Tatsuro; Inaba, Yusuke*; Takeshita, Kenji*
no journal, ,
no abstracts in English
Matsumura, Tatsuro; Ban, Yasutoshi; Hotoku, Shinobu; Suzuki, Hideya; Tsubata, Yasuhiro; Tsutsui, Nao; Morita, Keisuke; Toigawa, Tomohiro; Shibata, Mitsunobu*; Kurosawa, Tatsuya*; et al.
no journal, ,
no abstracts in English
Matsumura, Tatsuro; Ban, Yasutoshi; Hotoku, Shinobu; Suzuki, Hideya; Tsubata, Yasuhiro; Tsutsui, Nao; Morita, Keisuke; Toigawa, Tomohiro; Shibata, Mitsunobu*; Kurosawa, Tatsuya*; et al.
no journal, ,
no abstracts in English
Matsumura, Tatsuro; Ban, Yasutoshi; Hotoku, Shinobu; Suzuki, Hideya; Tsubata, Yasuhiro; Tsutsui, Nao; Morita, Keisuke; Toigawa, Tomohiro; Shibata, Mitsunobu*; Kurosawa, Tatsuya*; et al.
no journal, ,
no abstracts in English
Matsumura, Tatsuro; Ishii, Sho*; Suzuki, Akihiro*
no journal, ,
no abstracts in English
Ishii, Hiroto*; Oishi, Yuji*; Muta, Hiroaki*; Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Uno, Masayoshi*; Kurosaki, Ken*
no journal, ,
This study experimentally investigates the wettability of liquid cesium iodide on the stainless steel (SUS 316) solid surfaces by the sessile drop method. wettedly, and iodine compounds were revaporized by the oxidation of cesium iodide.