Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Chikashi; Nakajima, Kunihisa; Osaka, Masahiko
Journal of Nuclear Science and Technology, 59(3), p.345 - 356, 2022/03
Times Cited Count:1 Percentile:15.09(Nuclear Science & Technology)During a severe accident (SA) such as the Fukushima Daiichi Nuclear Power Plant accident, fission products (FP) can be retained on the surface of structural materials in reactors. Cesium (Cs) is an important FP, and various Cs compounds such as Cs silicates are formed on the surface of stainless steel (SS) in a reactor during a SA. We calculated total energies of Cs-Si-O compounds for evaluation on phase stability within an adiabatic approximation. The calculations indicate that Cs Si
Si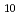 O
O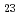 is the most stable of the Cs-Si-O compounds. We calculated, furthermore, total energies of Cs-Si-Fe-O compounds. These calculations indicate that Cs-Si-Fe-O compounds are more stable than C-Si-O compounds and that CsSi
is the most stable of the Cs-Si-O compounds. We calculated, furthermore, total energies of Cs-Si-Fe-O compounds. These calculations indicate that Cs-Si-Fe-O compounds are more stable than C-Si-O compounds and that CsSi FeO
FeO is the most stable of these C-Si-O and Cs-Si-Fe-O compounds within an adiabatic approximation. The results of our present calculations and our previous experiments lead to the conclusion that Cs-Si-Fe-O compounds can be stably formed on SS surface by Cs chemisorption.
is the most stable of these C-Si-O and Cs-Si-Fe-O compounds within an adiabatic approximation. The results of our present calculations and our previous experiments lead to the conclusion that Cs-Si-Fe-O compounds can be stably formed on SS surface by Cs chemisorption.
Rizaal, M.; Miwa, Shuhei; Suzuki, Eriko; Imoto, Jumpei; Osaka, Masahiko; Gou llo, M.*
llo, M.*
ACS Omega (Internet), 6(48), p.32695 - 32708, 2021/12
Times Cited Count:1 Percentile:13.70(Chemistry, Multidisciplinary)Miwa, Shuhei; Nakajima, Kunihisa; Suzuki, Chikashi; Rizaal, M.; Suzuki, Eriko; Horiguchi, Naoki; Osaka, Masahiko
Proceedings of Joint International Conference on Supercomputing in Nuclear Applications + Monte Carlo 2020 (SNA + MC 2020), p.253 - 260, 2020/12
We have proceeded the fundamental study for the improvement of the evaluation of fission product (FP) chemistry and the treatment of fine space resolution which are main issues for the evaluation of FP behaviors in a severe accident (SA). We have been developing FP chemistry database named ECUME for the improvement of SA analysis codes. We prepared thermodynamic data for Cs compounds which is no experimental data available by computational approach. Regarding the space resolution issue, we have been developing analysis tool named CHASER based on 3D-CFD code with the model for FP chemistry. More accurate evaluation of FP behavior can be achieved by incorporating ECUME to the CHASER.
 Si
Si O
O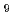
Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
Journal of Nuclear Science and Technology, 57(7), p.852 - 857, 2020/07
Times Cited Count:4 Percentile:43.68(Nuclear Science & Technology)The low temperature heat capacity of Cs Si
Si O
O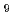 , which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity,
, which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity, 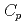
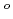 (298.15K), and the standard entropy,
(298.15K), and the standard entropy, 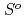 (298.15K), were 249.4
(298.15K), were 249.4  1.1 J K
1.1 J K mol
mol and 322.1
and 322.1  1.3 J K
1.3 J K mol
mol , respectively. The standard Gibbs energy of formation of Cs
, respectively. The standard Gibbs energy of formation of Cs Si
Si O
O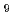 at high temperatures,
at high temperatures, 
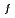
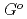 (
(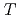 ), were reevaluated by using the presently obtained
), were reevaluated by using the presently obtained 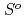 (298.15K) and the previously reported experimental results of the standard enthalpy of formation,
(298.15K) and the previously reported experimental results of the standard enthalpy of formation, 
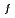
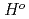 (298.15K), and the standard enthalpy increments at high temperatures,
(298.15K), and the standard enthalpy increments at high temperatures, 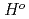 (
(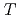 )-
)-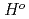 (298.15K).
(298.15K).
Nakajima, Kunihisa; Nishioka, Shunichiro*; Suzuki, Eriko; Osaka, Masahiko
Mechanical Engineering Journal (Internet), 7(3), p.19-00564_1 - 19-00564_14, 2020/06
A large amount of cesium (Cs) chemisorbed onto stainless steel is predicted to be present especially in the upper region of reactor pressure vessel (RPV) during light water reactor severe accident (LWR SA) and a chemisorption model was developed for estimation of such amounts of Cs for stainless steel type 304 (SS304). However, this existing chemisorption model cannot accurately reproduce experimental results. Therefore, in this study, a modified Cs chemisorption model which accounts for silicon content in SS304 and concentration of cesium hydroxide (CsOH) in gaseous phases was constructed by combining penetration theory for gas-liquid mass transfer with chemical reaction and mass action law for CsOH decomposition at interface between gaseous and solid phases. As a result, it was found that the modified model was able to reproduce the experimental data more accurately than the existing model.
Miwa, Shuhei; Nakajima, Kunihisa; Miyahara, Naoya; Nishioka, Shunichiro; Suzuki, Eriko; Horiguchi, Naoki; Liu, J.; Miradji, F.; Imoto, Jumpei; Afiqa, B. M.; et al.
Mechanical Engineering Journal (Internet), 7(3), p.19-00537_1 - 19-00537_11, 2020/06
We constructed the fission product (FP) chemistry database named ECUME for LWR severe accident. This version of ECUME is equipped with dataset of the chemical reactions and their kinetics constants for the reactions of cesium(Cs)-iodine(I)-boron(B)-molybdenum(Mo)-oxygen(O)-hydrogen(H) system in gas phase, the elemental model for the high temperature chemical reaction of Cs with stainless steel applied as the structural material in a reactor, and thermodynamic data for CsBO vapor species and solids of Cs
vapor species and solids of Cs Si
Si O
O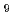 and CsFeSiO
and CsFeSiO for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
for these chemical reactions. The ECUME will provide estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:22 Percentile:81.39(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (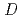
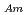 ) and Cm (
) and Cm (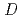
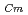 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
Miradji, F.; Suzuki, Chikashi; Nakajima, Kunihisa; Osaka, Masahiko
Journal of Physics and Chemistry of Solids, 136, p.109168_1 - 109168_9, 2020/01
Times Cited Count:2 Percentile:12.28(Chemistry, Multidisciplinary)Nishioka, Shunichiro; Nakajima, Kunihisa; Suzuki, Eriko; Osaka, Masahiko
Journal of Nuclear Science and Technology, 56(11), p.988 - 995, 2019/11
Times Cited Count:12 Percentile:79.16(Nuclear Science & Technology)In order to contribute to improvement of Cs chemisorption model used in severe accident analysis codes, the influence of chemical factors (temperature, atmosphere, concentration of affecting chemical elements etc.) on the Cs chemisorption behaviour onto stainless steel was investigated experimentally. It was found that the surface reaction rate constant used in the current Cs-chemisorption model was influenced by not only temperature, as already known, but also atmosphere, cesium hydroxide (CsOH) concentration in the gas phase and silicon content in SS304. Such chemical factors should be considered for the construction of the improved Cs-chemisorption model. Another important finding is that the chemisorption behavior at lower temperatures, around 873 K, could differ from those above 1073 K. Namely, Cs-Fe-O compounds would form as the main Cs-chemisorbed compounds at 873 K while Cs-Si-Fe-O compounds at more than 1073 K.
Miwa, Shuhei; Miyahara, Naoya; Nakajima, Kunihisa; Nishioka, Shunichiro; Suzuki, Eriko; Horiguchi, Naoki; Liu, J.; Miradji, F.; Imoto, Jumpei; Afiqa, B. M.; et al.
Proceedings of 27th International Conference on Nuclear Engineering (ICONE-27) (Internet), 8 Pages, 2019/05
We constructed the first version of fission product (FP) chemistry database named ECUME for LWR severe accident. The first version of ECUME is equipped with dataset of the chemical reactions and their kinetics constants for the reactions of cesium(Cs)-iodine(I)-boron(B)-molybdenum(Mo)-oxygen(O)-hydrogen(H) system in gas phase, the elemental model for the high temperature chemical reaction of Cs with stainless steel, and thermodynamic data for CsBO vapor species and solids of Cs
vapor species and solids of Cs Si
Si O
O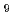 and CsFeSiO
and CsFeSiO . The ECUME will provide more accurate estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
. The ECUME will provide more accurate estimation of Cs distribution due to the evaluation of effects of interaction with BWR control material B and stainless steel on Cs behavior in the Fukushima Daiichi Nuclear Power Station.
Nakajima, Kunihisa; Nishioka, Shunichiro; Suzuki, Eriko; Osaka, Masahiko
Proceedings of 27th International Conference on Nuclear Engineering (ICONE-27) (Internet), 8 Pages, 2019/05
Cesium chemisorption models were developed for estimation of amount of cesium chemisorbed onto stainless steel type 304 (SS304) during light water reactor severe accident. However, existing chemisorption models cannot accurately reproduce experimental results. In this study, a modified cesium chemisorption model was constructed based on a penetration theory for gas-liquid mass transfer with chemical reaction and was able to adequately describe effects on concentration of cesium hydroxide in gaseous phase and silicon content in SS304. It was found that the modified model can more accurately reproduce the experimental data than the existing model.
Suzuki, Eriko; Takase, Gaku; Nakajima, Kunihisa; Nishioka, Shunichiro; Hashimoto, Naoyuki*; Isobe, Shigehito*; Osaka, Masahiko
Proceedings of International Topical Workshop on Fukushima Decommissioning Research (FDR 2019) (Internet), 4 Pages, 2019/05
In order to acquire the knowledge of the Cs chemisorption behaviour in the lower temperature region, the Cs chemisorbed compounds and the surface reaction rates were investigated by conducting the Cs chemisorption tests onto stainless steel at 873 and 973 K. As a result, The cesium ferrate compounds were revealed to be formed at this temperatures. It was seen that the dependences of surface reaction rate constant on this temperature were different from that at the higher temperature region. This behaviour leads to the conclusion that the Cs chemisorption model in the low temperature region should be newly constructed.
Mohamad, A.*; Nakajima, Kunihisa; Suzuki, Eriko; Miwa, Shuhei; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
Proceedings of International Topical Workshop on Fukushima Decommissioning Research (FDR 2019) (Internet), 4 Pages, 2019/05
In the accident of Fukushima Daiichi Nuclear Power Station, formation of a volatile SrCl could have occurred by the sea-water injection into the core. This can cause the release of non-volatile group Sr from the fuel to induce chemical reactions with reactor structural materials, such as stainless steel and Zircaloy (Zry) cladding. Such reactions could cause the changes in distribution of Sr in the reactor. Chemical reactions between Sr species and Zry were therefore investigated experimentally. As the result, it can be said that Sr vapor species were chemically trapped right after the release from fuel. This trapping effect of Sr by Zry-cladding implies a possibility of preferable Sr retention in the oxide phase of debris.
could have occurred by the sea-water injection into the core. This can cause the release of non-volatile group Sr from the fuel to induce chemical reactions with reactor structural materials, such as stainless steel and Zircaloy (Zry) cladding. Such reactions could cause the changes in distribution of Sr in the reactor. Chemical reactions between Sr species and Zry were therefore investigated experimentally. As the result, it can be said that Sr vapor species were chemically trapped right after the release from fuel. This trapping effect of Sr by Zry-cladding implies a possibility of preferable Sr retention in the oxide phase of debris.
Suzuki, Chikashi; Yaita, Tsuyoshi; Suzuki, Shinichi; Pacold, J.*; Altman, A. B.*; Minasian, S. G.*; Tyliszczak, T.*; Shuk, D. K.*; Yoshida, Hiroyuki; Osaka, Masahiko
Journal of Physics and Chemistry of Solids, 127, p.169 - 177, 2019/04
Times Cited Count:3 Percentile:15.75(Chemistry, Multidisciplinary)A combined method of NEXAFS measurement and DFT-calculation was employed for Cs evaluation in clay minerals. The Cs M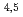 NEXAFS spectra of Cs halides were analyzed using the DFT-calculations, and were well reproduced by incorporating the core-hole strength. The Cs M
NEXAFS spectra of Cs halides were analyzed using the DFT-calculations, and were well reproduced by incorporating the core-hole strength. The Cs M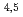 NEXAFS spectrum of clay minerals was well-reproduced by the DFT-calculations including the major transitions and tail structures with the established method. The further evaluation of this spectrum by charge density suggests that these major transitions and the tail structures likely reflect bonding states and local environments around the Cs atoms. Comparison of electronic states of Cs in the clay mineral with those in the Cs halides by DFT-calculations has shown that the interaction between Cs and the nearest-neighbor atom is largest in the clay mineral, because the energy level of Cs-5s and 5p is closer to that of O-2s and 2p than the s and p orbitals of other alkali metal and alkali earth metal elements.
NEXAFS spectrum of clay minerals was well-reproduced by the DFT-calculations including the major transitions and tail structures with the established method. The further evaluation of this spectrum by charge density suggests that these major transitions and the tail structures likely reflect bonding states and local environments around the Cs atoms. Comparison of electronic states of Cs in the clay mineral with those in the Cs halides by DFT-calculations has shown that the interaction between Cs and the nearest-neighbor atom is largest in the clay mineral, because the energy level of Cs-5s and 5p is closer to that of O-2s and 2p than the s and p orbitals of other alkali metal and alkali earth metal elements.
Miradji, F.; Suzuki, Chikashi; Nishioka, Shunichiro; Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko; Barrachin, M.*; Do, T. M. D.*; Murakami, Kenta*; Suzuki, Masahide*
Proceedings of 9th Conference on Severe Accident Research (ERMSAR 2019) (Internet), 21 Pages, 2019/03
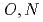 -hetero donor ligand BIZA
-hetero donor ligand BIZAKobayashi, Toru; Akutsu, Kazuhiro*; Nakase, Masahiko*; Suzuki, Shinichi; Shiwaku, Hideaki; Yaita, Tsuyoshi
Separation Science and Technology, 54(13), p.2077 - 2083, 2019/02
Times Cited Count:5 Percentile:21.84(Chemistry, Multidisciplinary)Development of separation reagent, which can efficiently separate lanthanides, is of increasing importance because it concerns establishment of purification and recycle techniques rare-earth elements. In our research group, several hetero donor type ligands, which have both oxygen and heterocyclic nitrogen atoms as donor atoms, were developed and revealed that this type of ligands show the selective complexation with specific lanthanide. In this paper, we will discuss the lanthaides complexation properties of the hetero donor type ligand containing benzimidazole group as nitrogen donor ( -methyl-
-methyl- -phenyl-2-(1
-phenyl-2-(1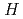 -benzimidazol-2-yl)-pyridine-6-carboxamide) based on structural investigation by crystallography.
-benzimidazol-2-yl)-pyridine-6-carboxamide) based on structural investigation by crystallography.
 -based simulated fuel containing CsI
-based simulated fuel containing CsITakamatsu, Yuki*; Ishii, Hiroto*; Oishi, Yuji*; Muta, Hiroaki*; Yamanaka, Shinsuke*; Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Kurosaki, Ken*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 17(3/4), p.106 - 110, 2018/12
In order to establish the synthesis method of simulated fuel contacting Cesium (Cs) which is required for the evaluation of physical/chemical characteristics in fuel and release behavior of Cs, sintering tests of the cerium dioxide (CeO ) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO
) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.
pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.

Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko
Progress in Nuclear Science and Technology (Internet), 5, p.165 - 167, 2018/11
In severe accident condition, CsFeSiO could be formed by Cesium (Cs) chemisorption onto reactor structural materials. For evaluation of re-vaporization behavior, effect of atmosphere on the vaporization behavior of CsFeSiO
could be formed by Cesium (Cs) chemisorption onto reactor structural materials. For evaluation of re-vaporization behavior, effect of atmosphere on the vaporization behavior of CsFeSiO at high temperature was investigated by thermal gravimetric-differential thermal analyzer (TG-DTA) experiments. As a result, it was found that vaporization of CsFeSiO
at high temperature was investigated by thermal gravimetric-differential thermal analyzer (TG-DTA) experiments. As a result, it was found that vaporization of CsFeSiO in reducing atmosphere (Ar-5%H
in reducing atmosphere (Ar-5%H ) started at relatively low temperature, about 800
) started at relatively low temperature, about 800 C, compared with in atmosphere containing H
C, compared with in atmosphere containing H O (Ar-5%H
O (Ar-5%H -5%H
-5%H O). It was inferred that a possible chemical reaction for the weight loss at around 800
O). It was inferred that a possible chemical reaction for the weight loss at around 800 C would occurred by the decomposition of CsFeSiO
C would occurred by the decomposition of CsFeSiO into volatile Cs vapor species under H
into volatile Cs vapor species under H .
.
Nakajima, Kunihisa; Suzuki, Eriko; Miyahara, Naoya; Osaka, Masahiko
Progress in Nuclear Science and Technology (Internet), 5, p.168 - 170, 2018/11
Chemical interaction between cesium (Cs) vapor and stainless steel (SS) surface known as chemisorption can cause a significant amount of Cs retention on the inner surfaces within the reactor pressure vessel during a light water reactor severe accident (SA). Although the chemisorption is known to be influenced with temperature and atmosphere, their dependancies are not yet fully understood. Therefore, the Cs chemisorption behaviours under mixed gases of steam and hydrogen were experimentally examined to contribute to a better understanding of the chemisorption behaviours under various atmospheres experienced during the SA at the Fukushima Daiichi Nuclear Power Station. As a result, it was found that the deposited amounts of Cs onto the SS in the steam-containing atmosphere were much higher than those in the no steam atmosphere. It was considered that Cs revaporization from a chemisorbed product was one of the reasons why the deposited amounts under the reducing atmosphere decreased.
Kobata, Masaaki; Okane, Tetsuo; Nakajima, Kunihisa; Suzuki, Eriko; Owada, Kenji; Kobayashi, Keisuke*; Yamagami, Hiroshi; Osaka, Masahiko
Journal of Nuclear Materials, 498, p.387 - 394, 2018/01
Times Cited Count:18 Percentile:87.53(Materials Science, Multidisciplinary)In this study, for the understandings of Cesium (Cs) adsorption behavior on structure materials in severe accidents at a light water nuclear reactor, the chemical state of Cs and its distribution on the surface of SUS304 stainless steel (SS) with different Si concentration were investigated by hard X-ray photoelectron spectroscopy (HAXPES) and scanning electron microscope / energy dispersive X-ray spectroscopy (SEM/EDX). As a result, it was found that Cs is selectively adsorbed at the site where Si distributes with high concentration. CsFeSiO is a dominant Cs products in the case of low Si content, mainly formed, while Cs
is a dominant Cs products in the case of low Si content, mainly formed, while Cs Si
Si O
O and Cs
and Cs Si
Si O
O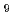 are formed in addition to CsFeSiO
are formed in addition to CsFeSiO in the case of high Si content. The chemical forms of the Cs compounds produced in the adsorption process on the SS surface has a close correlation with the concentration and chemical states of Si originally included in SS.
in the case of high Si content. The chemical forms of the Cs compounds produced in the adsorption process on the SS surface has a close correlation with the concentration and chemical states of Si originally included in SS.