Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
粟飯原 はるか; 渡部 創; 柴田 淳広; Mahardiani, L.*; 大友 亮一*; 神谷 裕一*
Progress in Nuclear Energy, 139, p.103872_1 - 103872_9, 2021/09
被引用回数:2 パーセンタイル:30.55(Nuclear Science & Technology)To prevent unexpected accidents at nuclear facilities caused by accumulated ammonium nitrate in an aqueous liquid waste containing ammonium salts and nitric acid, NH 
 in the liquid waste must be decomposed under mild reaction conditions. In this study, we investigated the oxidative decomposition of NH
in the liquid waste must be decomposed under mild reaction conditions. In this study, we investigated the oxidative decomposition of NH 
 with O
with O at 333 K in the presence of a homogeneous Co
at 333 K in the presence of a homogeneous Co catalyst and Cl
catalyst and Cl in the wide pH range of the test solution. The reaction behavior was greatly affected by pH of the test solution. In a basic solution at pH 12, high conversion of NH
in the wide pH range of the test solution. The reaction behavior was greatly affected by pH of the test solution. In a basic solution at pH 12, high conversion of NH 
 was obtained even in the absence of Co
was obtained even in the absence of Co and Cl
and Cl and the main product was NO
and the main product was NO 
 . However, Co
. However, Co and Cl
and Cl in the solution greatly enhanced the decomposition rate of NH
in the solution greatly enhanced the decomposition rate of NH 
 in acidic to mild basic solutions (pH 1-8), while only low conversion of NH
in acidic to mild basic solutions (pH 1-8), while only low conversion of NH 
 was observed unless both Co
was observed unless both Co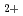 and Cl
and Cl were present. For the reaction with Co
were present. For the reaction with Co and Cl
and Cl in the solutions, NH
in the solutions, NH
 was transformed mainly into chloramines (NH
was transformed mainly into chloramines (NH  Cl
Cl  , x = 1-3) by the reaction with HClO, which was formed by the reaction of Cl
, x = 1-3) by the reaction with HClO, which was formed by the reaction of Cl with O
with O catalyzed by the homogeneous Co
catalyzed by the homogeneous Co catalyst, and led to the high decomposition rate of NH
catalyst, and led to the high decomposition rate of NH
 . Cl
. Cl suppressed the formation of the precipitate CoO(OH) during the reaction and consequently the Co
suppressed the formation of the precipitate CoO(OH) during the reaction and consequently the Co catalyst stably existed in the reaction solution, which was another reason for the high decomposition rate of NH
catalyst stably existed in the reaction solution, which was another reason for the high decomposition rate of NH
 in the presence of Cl
in the presence of Cl . Owing to the swift decomposition of NH
. Owing to the swift decomposition of NH
 under mild reaction conditions and small formation of secondary waste, the oxidative decomposition of NH
under mild reaction conditions and small formation of secondary waste, the oxidative decomposition of NH
 in the presence of the homogeneous Co
in the presence of the homogeneous Co catalyst and Cl
catalyst and Cl is suitable and applicable for the treatment of the aqueous liquid waste containing ammonium salts and nitric acid.
is suitable and applicable for the treatment of the aqueous liquid waste containing ammonium salts and nitric acid.