Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Iwai, Yasunori; Kubo, Hitoshi*; Oshima, Yusuke*
Isotope News, (736), p.12 - 17, 2015/08
We have successfully developed a new hydrophobic platinum catalyst for collecting tritium at nuclear fusion reactors. Catalysts used to collect tritium are called hydrophobic precious metal catalysts. In Japan, hydrophobic precious metal catalysts manufactured from polymers have been used for heavy water refinement.However, this catalyst has issues related to embrittlement to radiation and thermal stability. These technological issues needed to be solved to allow for its application to nuclear fusion reactors requiring further enrichment from highly-concentrated tritiated water. We developed a new method of manufacturing catalysts involving hydrophobic processing with an inorganic substance base. As a result, previous technological issues were able to be solved with the development of a catalyst that exhibited no performance degradation in response to radiation application of 530kGy, a standard for radiation resistance, and maintenance of thermal stability at over 600 C, which is much higher than the 70
C, which is much higher than the 70 C temperature that is normally used. The catalyst created with this method was also confirmed to have achieved the world's highest exchange efficiency, equivalent to 1.3 times the previously most powerful efficiency. The application of this catalyst to the liquid phase catalytic exchange process is expected to overcome significant technological hurdles with regards to improving the reliability and efficiency of systems for collecting tritium from tritiated water.
C temperature that is normally used. The catalyst created with this method was also confirmed to have achieved the world's highest exchange efficiency, equivalent to 1.3 times the previously most powerful efficiency. The application of this catalyst to the liquid phase catalytic exchange process is expected to overcome significant technological hurdles with regards to improving the reliability and efficiency of systems for collecting tritium from tritiated water.
Iwai, Yasunori; Kubo, Hitoshi*; Oshima, Yusuke*
Kagaku, 70(5), p.35 - 40, 2015/05
We have successfully developed a new hydrophobic platinum catalyst for collecting tritium at nuclear fusion reactors. Catalysts used to collect tritium are called hydrophobic precious metal catalysts. In Japan, hydrophobic precious metal catalysts manufactured from polymers have been used for heavy water refinement. However, this catalyst has issues related to embrittlement to radiation and thermal stability. These technological issues needed to be solved to allow for its application to nuclear fusion reactors requiring further enrichment from highly-concentrated tritiated water. We developed a new method of manufacturing catalysts involving hydrophobic processing with an inorganic substance base. As a result, previous technological issues were able to be solved with the development of a catalyst that exhibited no performance degradation in response to radiation application of 530 kGy, a standard for radiation resistance, and maintenance of thermal stability at over 600 C, which is much higher than the 70
C, which is much higher than the 70 C temperature that is normally used. The catalyst created with this method was also confirmed to have achieved the world's highest exchange efficiency, equivalent to 1.3 times the previously most powerful efficiency. The application of this catalyst to the liquid phase catalytic exchange process is expected to overcome significant technological hurdles with regards to improving the reliability and efficiency of systems for collecting tritium from tritiated water.
C temperature that is normally used. The catalyst created with this method was also confirmed to have achieved the world's highest exchange efficiency, equivalent to 1.3 times the previously most powerful efficiency. The application of this catalyst to the liquid phase catalytic exchange process is expected to overcome significant technological hurdles with regards to improving the reliability and efficiency of systems for collecting tritium from tritiated water.
Seko, Noriaki; Takeda, Hayato*; Kasai, Noboru; Tamada, Masao; Hasegawa, Shin; Katakai, Akio; Sugo, Takanobu*
JAERI-Tech 2004-075, 51 Pages, 2005/01
Fibrous adsorbent which is synthesized by radiation induced graft polymerization on the trunk polymers such as polymer nonwoven fabrics and woven cloths exhibits an excellent selective adsorption against heavy metal ions and toxic gases at extremely low concentrations. Two equipments were installed to synthesize the metal-ion and gas adsorbents by means of the radiation-induced graft polymerization in the liquid phase and the dipping, respectively. In the reaction chamber of the liquid phase reactor, the oxygen decreased to 100ppm. The inside temperature raised to 80 C. These characteristics satisfied the specification. The fabric transport can regulate the rate in the range from 1 to 10m/min. The reactor for the dip grafting could reduce the inside oxygen to 100ppm and inside temperature could reach to 80
C. These characteristics satisfied the specification. The fabric transport can regulate the rate in the range from 1 to 10m/min. The reactor for the dip grafting could reduce the inside oxygen to 100ppm and inside temperature could reach to 80  C. The grafting of GMA was carried out as a characteristic test. The degree of grafting was controlled in the range from 40 to 70%.
C. The grafting of GMA was carried out as a characteristic test. The degree of grafting was controlled in the range from 40 to 70%.

Fuchizaki, Kazuhiro*; Fujii, Yasuhiko*; Oishi, Yasuo*; Omura, Ayako*; Hamaya, Nozomu*; Katayama, Yoshinori; Okada, Taku
Journal of Chemical Physics, 120(23), p.11196 - 11199, 2004/06
Times Cited Count:24 Percentile:60.17(Chemistry, Physical)The location of the liquidus in the low-pressure crystalline phase of SnI was determined utilizing
was determined utilizing  X-ray diffraction measurements under pressures up to approximately
X-ray diffraction measurements under pressures up to approximately 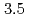 GPa. The liquidus is not well fitted to a monotonically increasing curve such as Simon's equation, but breaks near
GPa. The liquidus is not well fitted to a monotonically increasing curve such as Simon's equation, but breaks near  GPa and then becomes almost flat. The results are compared to those from molecular dynamics simulations. Ways to improve the model potential adopted in the simulations are discussed.
GPa and then becomes almost flat. The results are compared to those from molecular dynamics simulations. Ways to improve the model potential adopted in the simulations are discussed.
Shiina, Yasuaki; Inagaki, Terumi*
Nihon Kikai Gakkai Rombunshu, B, 69(681), p.1233 - 1241, 2003/05
In order to reduce phase change time in latent heat technology, improvement of effective thermal conductivity of heat storage unit would be one of the techniques. Effect of effective thermal conductivity on melting time are studied analytically of circular composite heat storage capsules made by immersing phase change materials (PCM) into porous metals. Numerical and approximate analysis were made with the consideration of uniform and non-uniform heat transfer coefficients around the cylindrical surface. Four PCMs (H O,Octadecane, Li
O,Octadecane, Li CO
CO , NaCl) and three metals (copper, aluminum and carbon steel) were selected specific materials. Porosity of the metals were restricted larger than 0.9 in order to lessen decrease in latent heat. Results show that reduction in melting time was obtained for the above PCMs, especially for low conductivity PCMs. Melting time obtained by approximate analysis agrees well with numerical analysis. High Nusselt number and high thermal conductivity of heat transfer fluid would be more effective to reduce phase change time.
, NaCl) and three metals (copper, aluminum and carbon steel) were selected specific materials. Porosity of the metals were restricted larger than 0.9 in order to lessen decrease in latent heat. Results show that reduction in melting time was obtained for the above PCMs, especially for low conductivity PCMs. Melting time obtained by approximate analysis agrees well with numerical analysis. High Nusselt number and high thermal conductivity of heat transfer fluid would be more effective to reduce phase change time.
Chikazumi, Shimpei*; Maruyama, Toshiki; Niita, Koji*; Iwamoto, Akira
Physics Letters B, 476(3-4), p.273 - 278, 2000/03
Times Cited Count:10 Percentile:51.27(Astronomy & Astrophysics)no abstracts in English
; Shiina, Yasuaki; Inagaki, Terumi*
Kashika Joho Gakkai-Shi, 19(75), p.41 - 45, 1999/10
no abstracts in English
 CO
CO , MgCl
, MgCl and CaCl
and CaCl as heat storage medium
as heat storage mediumShiina, Yasuaki
JAERI-Tech 98-056, 64 Pages, 1998/12
no abstracts in English
Kato, Yoshiharu; Kimura, Takaumi; Yoshida, Zenko; Shirasu, Noriko
Radiochimica Acta, 82, p.63 - 68, 1998/00
no abstracts in English
Kimura, Takaumi; ; Yoshida, Zenko; Shirasu, Noriko
Journal of Nuclear Science and Technology, 33(6), p.519 - 521, 1996/06
no abstracts in English
 partial pressures
partial pressures; Kimura, Takaumi; Yoshida, Zenko;
Radiochimica Acta, 74, p.21 - 25, 1996/00
no abstracts in English
Yamanishi, Toshihiko; Iwai, Yasunori; Okuno, Kenji
JAERI-Research 95-058, 22 Pages, 1995/09
no abstracts in English
; ;
J.Chem.Eng.Jpn., 16(6), p.513 - 516, 1983/00
Times Cited Count:5 Percentile:69.53(Engineering, Chemical)no abstracts in English
; ; ;
Zairyo, 26(288), p.863 - 867, 1977/00
no abstracts in English
Ugajin, Mitsuhiro; Abe, Jiro;
Journal of Nuclear Science and Technology, 12(9), p.560 - 566, 1975/09
Times Cited Count:4no abstracts in English
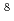
 Kr dissolved in the soution, 1; Methods and effects of removal
Kr dissolved in the soution, 1; Methods and effects of removal; Yamamoto, Tadatoshi
Nihon Genshiryoku Gakkai-Shi, 16(2), p.98 - 104, 1974/02
no abstracts in English
Kaetsu, Isao; ;
J.Appl.Polym.Sci., 17(9), p.2743 - 2752, 1973/09
Times Cited Count:3no abstracts in English