Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakamura, Satoshi; Suzuki, Hideya*; Ban, Yasutoshi; Ohashi, Akira*
Progress in Nuclear Science and Technology (Internet), 8, p.228 - 232, 2025/09
To reduce volume and radiotoxicity of high-level radioactive waste, JAEA has been developing a separation process recovering minor actinides (MA) from high-level radioactive liquid-waste. In the separation process, separating trivalent MA such as Am and Cm, from RE is challenging due to their similar chemical properties. In this study, extraction properties of three different glycine-based amic-acid-type extractants for MA(III) and RE(III) were studied by a single-stage batch method. The results revealed that the distribution ratios of all metal ions increased with an increase of equilibrium pH, and all extractants showed higher distribution ratios for Am than for RE.
Yamaguchi, Toshio*; Machida, Shinichi*; Hattori, Takanori
Molecules (Internet), 30(16), p.3417_1 - 3417_18, 2025/08
Times Cited Count:0 Percentile:0.001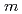 (mol/kg) ScCl
(mol/kg) ScCl aqueous solution in D
aqueous solution in D O was investigated under the thermodynamic conditions from 0.1 MPa/298 K to 4 GPa/523 K by in-situ neutron diffraction coupled with the empirical potential structure refinement (EPSR) method. A predominant Sc(III) species is [Sc(OH
O was investigated under the thermodynamic conditions from 0.1 MPa/298 K to 4 GPa/523 K by in-situ neutron diffraction coupled with the empirical potential structure refinement (EPSR) method. A predominant Sc(III) species is [Sc(OH )
)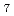 ]
] with a distorted pentagonal bipyramidal geometry together with appreciable amounts of contact ion pair species [ScCl
with a distorted pentagonal bipyramidal geometry together with appreciable amounts of contact ion pair species [ScCl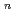 (OH
(OH )
)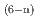 ]
]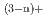 (n=1-3) and [Sc(OH
(n=1-3) and [Sc(OH )
) ]
] with mean Sc-Cl and Sc-OH
with mean Sc-Cl and Sc-OH distances of 2.42 and 2.11
distances of 2.42 and 2.11 O distance of 3.10
O distance of 3.10  at 0.1 MPa/298 K and 4 GPa/523 K, respectively. Applying GPa pressure transforms the tetrahedral network structure of water under ambient conditions to a dense, randomly packed structure with a mean coordination number of 12.6, resulting in an increase in the first-neighbor distance from 2.77 to 2.89 AA. The hydrogen bonds between water molecules remain linear but are largely distorted at high temperatures and high pressures.
at 0.1 MPa/298 K and 4 GPa/523 K, respectively. Applying GPa pressure transforms the tetrahedral network structure of water under ambient conditions to a dense, randomly packed structure with a mean coordination number of 12.6, resulting in an increase in the first-neighbor distance from 2.77 to 2.89 AA. The hydrogen bonds between water molecules remain linear but are largely distorted at high temperatures and high pressures.
Ueda, Yuki; Micheau, C.; Motokawa, Ryuhei
Fuain Kemikaru, 54(5), p.53 - 60, 2025/05
no abstracts in English
Suzuki, Hideya*; Ban, Yasutoshi
Journal of Nuclear Science and Technology, 62(2), p.157 - 166, 2025/02
Times Cited Count:1 Percentile:35.03(Nuclear Science & Technology)Ito, Kengo*; Takahashi, Shin*; Kato, Chizu*; Fukutani, Satoshi*; Matsumura, Tatsuro; Fujii, Toshiyuki*
Journal of Radioanalytical and Nuclear Chemistry, 334, p.2467 - 2475, 2025/02
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)In this study, the solvent extraction behavior of tin (Sn), specifically  Sn, from high-level radioactive waste was evaluated using six different extractants in a HNO
Sn, from high-level radioactive waste was evaluated using six different extractants in a HNO system. Among the tested extractants, N,N-Didodecyl-2-hydroxyacetoamide (HAA) exhibited higher efficiency, still not sufficient for industrial implementation. In systems where HCl was added to HNO
system. Among the tested extractants, N,N-Didodecyl-2-hydroxyacetoamide (HAA) exhibited higher efficiency, still not sufficient for industrial implementation. In systems where HCl was added to HNO , both tributyl phosphate (TBP) and N,N,N,N'-tetra-2-ethylhexyl diglycolamide (TEHDGA) achieved D
, both tributyl phosphate (TBP) and N,N,N,N'-tetra-2-ethylhexyl diglycolamide (TEHDGA) achieved D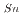 values greater than 1 at
values greater than 1 at  1 M HCl. However, due to practical challenges in industrial applications, HAA extraction in HNO
1 M HCl. However, due to practical challenges in industrial applications, HAA extraction in HNO systems, particularly at low Sn concentrations (0.0008 M), may provide a more effective solution for Sn recovery.
systems, particularly at low Sn concentrations (0.0008 M), may provide a more effective solution for Sn recovery.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Suzuki, Tatsuya*
Solvent Extraction Research and Development, Japan, 32(1), p.21 - 29, 2025/00
The magnitude of the masking effect of carboxylic, amic-acidic, and amidic compounds through Ln and Am extractions by tetraoctyl diglycolamide (TODGA) is compared. The compounds used are diglycol, ethylenediamine, diethylenetriamine-type, and two other amides (dioxaoctane diamide and nitrylotriacetamide). The results show that below pH 1.2, where carboxylic acids are less dissociated, amide O atoms have higher reactivity with lanthanides than O atoms in carboxyl groups. From observing the Ln patterns (D(Ln) vs. their atomic number), the compounds primarily show high reactivity, with middle and heavy Ln having a higher charge density than light Ln. Four amide compounds are employed in this work. Those with tertiary amine N atoms have pH dependence on D(Ln) due to protonation and dissociation from amine N atoms.
Ito, Kengo*; Morita, Misaki*; Araki, Yuta*; Kato, Chizu*; Fukutani, Satoshi*; Matsumura, Tatsuro; Fujii, Toshiyuki*
Solvent Extraction Research and Development, Japan, 32(1), p.53 - 62, 2025/00
Rhodium (Rh) and Palladium (Pd) in high-level radioactive waste are primarily fission products. This Study focused on understanding the extraction behavior of these platinum group elements (PGEs) using the novel extractants  -hexaoctylnitriloacetamide (HONTA) and alkyl diamideamine (ADAAM). Both extractants showed affinity for Pd, with distribution coefficients significantly exceeding 1, demonstrating their effectiveness in Pd separation. In contrast, the distribution coefficients for Rh were consistently below 10
-hexaoctylnitriloacetamide (HONTA) and alkyl diamideamine (ADAAM). Both extractants showed affinity for Pd, with distribution coefficients significantly exceeding 1, demonstrating their effectiveness in Pd separation. In contrast, the distribution coefficients for Rh were consistently below 10  , indicating low extraction efficiency from nitric acid. However, by leveraging the salting-out effect with calcium nitrate hydrate, a distribution coefficient of
, indicating low extraction efficiency from nitric acid. However, by leveraging the salting-out effect with calcium nitrate hydrate, a distribution coefficient of  570 for Rh was achieved using HONTA. To overcome the difficult back-extraction of PGEs with HONTA, experiments were conducted using HEDTA and thiourea. Back-extraction with HEDTA in high-concentration nitric acid (
570 for Rh was achieved using HONTA. To overcome the difficult back-extraction of PGEs with HONTA, experiments were conducted using HEDTA and thiourea. Back-extraction with HEDTA in high-concentration nitric acid ( 2M) resulted in
2M) resulted in  90% extraction of Pd, while thiourea-based back-extraction with nitric acid yielded over 40% extraction for Rh, with the maximum of 62.7% achieved using hydrochloric acid.
90% extraction of Pd, while thiourea-based back-extraction with nitric acid yielded over 40% extraction for Rh, with the maximum of 62.7% achieved using hydrochloric acid.
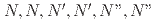 -hexahexyl-nitrilotriacetamide and electrodeposition
-hexahexyl-nitrilotriacetamide and electrodepositionMatsumiya, Masahiko*; Tokumitsu, Shun*; Mishima, Takumi*; Sasaki, Yuji
ECS Advances (Internet), 3(4), p.043001_1 - 043001_8, 2024/12
The extraction behavior of Rh(III) with Hexahexyl-nitrilotriacetamide, NTAamide(C6) was investigated in three different diluents (acetophenone, AP, 1,2-dichloroethane, DCE, and 1-octanol, OC). The electrochemical behavior of the extracted Rh(III) complex in each diluent was investigated from linear sweep voltammetry. It was revealed that Rh(III) was reduced to Rh(0) metal by a three electron transfer in NTAamide(C6)/AP, DCE and OC system. The electrodeposits can be recovered from continuous solvent extraction and direct electrodeposition. The electrodeposits were identified by XPS and XRD analyses as mainly Rh metal.
Ueda, Yuki; Micheau, C.; Akutsu, Kazuhiro*; Tokunaga, Kohei; Yamada, Masako*; Yamada, Norifumi*; Bourgeois, D.*; Motokawa, Ryuhei
Langmuir, 40(46), p.24257 - 24271, 2024/11
Times Cited Count:1 Percentile:16.77(Chemistry, Multidisciplinary)Microscopic structures in liquid-liquid extraction, such as structuration between extractants or extracted complexes in bulk organic phases and at interfaces, can influence macroscopic phenomena, such as the distribution behavior of solutes, including extraction efficiency, selectivity, and phase separation of the organic phase. In this study, we correlated the macroscopic behavior of the extraction of Zr(IV) ions from nitric acid solutions with microscopic structural information in order to understand at the molecular level the key factors contributing to the higher metal ion extraction performance in the fluorous extraction system comprising fluorous phosphate (TFP) in perfluorohexane as compared to the analogous organic extraction system comprising organic phosphate (THP) in n-hexane. Extended X-ray absorption fine structure, neutron reflectometry, and small-angle neutron scattering revealed the local coordination structure around the Zr(IV) ion, the accumulation of extractant molecules at the interface, and the structuration of extractant molecules in the bulk extraction phase, respectively. In the fluorous extraction system, extractant aggregates with were formed after contact with nitric acid. In contrast, in the organic extraction system, only extractant dimers were formed. We speculate that differences in the local coordination structure around the Zr(IV) ion and the structuration of the extractant molecules in the bulk extraction phase contribute to the high Zr(IV) extraction performance in the fluorous extraction system. In particular, the size of the aggregates hardly changed with increasing Zr(IV) concentration in the fluorous phase, which may be closely related to the absence of phase splitting in the fluorous extraction system.
 Mo/
Mo/ Tc radioisotope separation by modified extraction method
Tc radioisotope separation by modified extraction methodPratama, C.*; Fujita, Yoshitaka; Saptiama, I.*; Marlina, M.*; Triyatna, F.*; Ilhami, A.*; Maria, C. P.*; Tsuchiya, Kunihiko; Teguh, A.*; Prasetyo, I.*
Journal of Physics; Conference Series, 2828, p.012025_1 - 012025_12, 2024/10
no abstracts in English
Ito, Kengo*; Kawakami, Takahiro*; Kato, Chizu*; Fukutani, Satoshi*; Matsumura, Tatsuro; Fujii, Toshiyuki*
Journal of Radioanalytical and Nuclear Chemistry, 333(10), p.5183 - 5189, 2024/10
Times Cited Count:1 Percentile:35.03(Chemistry, Analytical)Solvent extraction behaviors of Se (VI) from nitric acid solutions were investigated with multiple extractants used for uranium, plutonium, minor actinides, and rare earthelements separation processes from high-level liquid waste. During the processes, Se remained in the residual aqueous solutions, as all extractants showed distributionratios  1. In contrast, Se showed distribution ratios
1. In contrast, Se showed distribution ratios  1 with
1 with 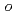 -phenylenediamine in dilute nitric acid (
-phenylenediamine in dilute nitric acid ( 2 M HNO
2 M HNO ), octanol as the organic phase, and concentrated nitric acid (8 M HNO
), octanol as the organic phase, and concentrated nitric acid (8 M HNO ) for back extraction, suggesting a potential new single separation process and recovery of Se.
) for back extraction, suggesting a potential new single separation process and recovery of Se.
Ban, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Proceedings of International Conference on Nuclear Fuel Cycle (GLOBAL2024) (Internet), 4 Pages, 2024/10
A continuous counter-current extraction experiment was performed by mixer-settler extractors to recover minor actinides (MA; Am and Cm) from high-level liquid waste. Using hexaoctyl nitrilotriacetamide (HONTA) as an extractant, 0.17 g of MA was recovered in a MA fraction.
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by  -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Nakagawa, Taichi; Suzuki, Reika*; Matsueda, Makoto; Terashima, Motoki; Hinze, W. L.*; Takagai, Yoshitaka*
Solvent Extraction Research and Development, Japan, 31(2), p.49 - 56, 2024/00
 solution in the gigapascal pressure range
solution in the gigapascal pressure rangeYamaguchi, Toshio*; Fukuyama, Nami*; Yoshida, Koji*; Katayama, Yoshinori*; Machida, Shinichi*; Hattori, Takanori
Liquids (Internet), 3(3), p.288 - 302, 2023/09
We report the structure of an aqueous 2 mol/kg MgCl solution at pressures from 0.1 MPa to 4 GPa and temperatures from 300 to 500 K revealed by X-ray and neutron scattering measurements. The scattering data are analyzed by empirical potential structure refinement (EPSR) modeling to derive the pair distribution functions, coordination number distributions, angle distributions, and spatial density functions as a function of pressure and temperature. Mg
solution at pressures from 0.1 MPa to 4 GPa and temperatures from 300 to 500 K revealed by X-ray and neutron scattering measurements. The scattering data are analyzed by empirical potential structure refinement (EPSR) modeling to derive the pair distribution functions, coordination number distributions, angle distributions, and spatial density functions as a function of pressure and temperature. Mg forms rigid solvation shells extended to the third shell; the first solvation shell of six-fold octahedral coordination with about six water molecules at 0 GPa transforms into about five water molecules and one Cl
forms rigid solvation shells extended to the third shell; the first solvation shell of six-fold octahedral coordination with about six water molecules at 0 GPa transforms into about five water molecules and one Cl due to the formation of the contact ion pairs in the GPa pressure range. The Cl
due to the formation of the contact ion pairs in the GPa pressure range. The Cl solvation shows a substantial pressure dependence; the coordination number of a water oxygen atom around Cl
solvation shows a substantial pressure dependence; the coordination number of a water oxygen atom around Cl increases from 8 at 0.1 MPa/300 K to 10 at 4 GPa/500 K. The solvent water transforms the tetrahedral network structure at 0.1 MPa/300 K to a densely packed structure in the GPa pressure range; the number of water oxygen atoms around a central water molecule gradually increases from 4.6 at 0.1 MPa/298 K to 8.4 at 4 GPa/500 K.
increases from 8 at 0.1 MPa/300 K to 10 at 4 GPa/500 K. The solvent water transforms the tetrahedral network structure at 0.1 MPa/300 K to a densely packed structure in the GPa pressure range; the number of water oxygen atoms around a central water molecule gradually increases from 4.6 at 0.1 MPa/298 K to 8.4 at 4 GPa/500 K.
Suzuki, Hideya*; Ban, Yasutoshi
Analytical Sciences, 39(8), p.1341 - 1348, 2023/08
Times Cited Count:5 Percentile:46.16(Chemistry, Analytical)The Japan Atomic Energy Agency (JAEA) has proposed the Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation (SELECT) process by solvent extraction as a new separation technology to recover minor actinides (MA) from high-level liquid waste (HLLW) produced by spent fuel reprocessing. The MA separation in the SELECT process comprises the batch recovery of MA and rare earths (RE) from HLLW, MA/RE separation, and Am/Cm separation. Three highly practical extractants are used in the MA separation. Furthermore, this flow configuration facilitates the preparation of nitric acid concentrations in the aqueous phase. However, the separation factor between Cm and Nd in the MA/RE separation is small (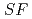
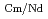 = 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
= 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
 Tc separation/concentration technology from
Tc separation/concentration technology from  Mo by (n,
Mo by (n,  ) method
) methodFujita, Yoshitaka; Hu, X.*; Takeuchi, Tomoaki; Takeda, Ryoma; Fujihara, Yasuyuki*; Yoshinaga, Hisao*; Hori, Junichi*; Suzuki, Tatsuya*; Suematsu, Hisayuki*; Ide, Hiroshi
KURNS Progress Report 2022, P. 110, 2023/07
no abstracts in English
Nuclear Science and Engineering Center; Fuel Cycle Design Office; Plutonium Fuel Development Center; Nuclear Plant Innovation Promotion Office; Fast Reactor Cycle System Research and Development Center; J-PARC Center
JAEA-Review 2022-052, 342 Pages, 2023/02
This report summarizes the current status and future plans of research and development (R&D) on partitioning and transmutation technology in Japan Atomic Energy Agency, focusing on the results during the 3rd Medium- to Long-term Plan period (FY 2015-2021). Regarding the partitioning technology, R&D of the solvent extraction method and the extraction chromatography method are described, and regarding the minor actinide containing fuel technology, R&D of the oxide fuel production using the simplified pellet method, the nitride fuel production using the external gelation method, and pyrochemical reprocessing of the nitride fuel were summarized. Regarding transmutation technology, R&D of technology using fast reactors and accelerator drive systems were summarized. Finally, the new facilities necessary for the future R&D were mentioned.
Micheau, C.; Ueda, Yuki; Akutsu, Kazuhiro*; Bourgeois, D.*; Motokawa, Ryuhei
Solvent Extraction and Ion Exchange, 41(2), p.221 - 240, 2023/02
Times Cited Count:4 Percentile:33.15(Chemistry, Multidisciplinary)Uchino, Seiko*; Narita, Hirokazu*; Kita, Keisuke*; Suzuki, Hideya*; Matsumura, Tatsuro; Naganawa, Hirochika*; Sakaguchi, Koichi*; Oto, Keisuke*
Solvent Extraction Research and Development, Japan, 30(1), p.39 - 46, 2023/00
The extraction of trivalent rare earth ions (RE ) from HNO
) from HNO solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO
solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO and RE
and RE and the relationship between atomic number and extraction percentages (E%) for RE
and the relationship between atomic number and extraction percentages (E%) for RE . A DEHTAA molecule dominantly formed a DEHTAA HNO
. A DEHTAA molecule dominantly formed a DEHTAA HNO at 1.0 M HNO
at 1.0 M HNO and a DEHTAA(HNO
and a DEHTAA(HNO )
) at 6.0 M HNO
at 6.0 M HNO in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE
in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE on the HNO
on the HNO concentration, in which the E% value had a minimum and maximum at
concentration, in which the E% value had a minimum and maximum at  0.5 M and
0.5 M and  2 M HNO
2 M HNO , respectively. The results of the slope analyses for the distribution ratios for RE
, respectively. The results of the slope analyses for the distribution ratios for RE suggested that the dominant RE
suggested that the dominant RE complex was RE(NO
complex was RE(NO )
) DEHTAA(DEHTAA HNO
DEHTAA(DEHTAA HNO ) at 1.0 M HNO
) at 1.0 M HNO . The E% for RE
. The E% for RE decreased from La
decreased from La to Lu
to Lu at 1.0 M HNO
at 1.0 M HNO ; on the other hand, those increased from La
; on the other hand, those increased from La to Nd
to Nd at 0.25 M and from La
at 0.25 M and from La to Sm
to Sm and 6.0 M HNO
and 6.0 M HNO .
.