Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Soma, Yasutaka; Komatsu, Atsushi; Kaji, Yoshiyuki; Yamamoto, Masahiro*; Igarashi, Takahiro
Corrosion Science, 251, p.112897_1 - 112897_15, 2025/07
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Experimental and modeling studies of the oxygen ingression at the crevices of stainless steels were conducted in high-temperature water (288 C). The limiting distance of oxygen ingression,
C). The limiting distance of oxygen ingression, 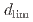 , was defined as the point beyond which the primary surface oxide changed (hematite
, was defined as the point beyond which the primary surface oxide changed (hematite  magnetite), regardless of crevice gap, oxygen concentration, and time. In situ measurements revealed increased electrical conductivity around the
magnetite), regardless of crevice gap, oxygen concentration, and time. In situ measurements revealed increased electrical conductivity around the 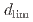 position indicating ion enrichment due to a differential oxygen concentration cell.
position indicating ion enrichment due to a differential oxygen concentration cell. 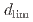 increased with increasing crevice gap, oxygen concentration, and immersion time. Modeling study suggested that oxide layer growth reduced anodic dissolution and slowed oxygen consumption, allowing oxygen ingression with time.
increased with increasing crevice gap, oxygen concentration, and immersion time. Modeling study suggested that oxide layer growth reduced anodic dissolution and slowed oxygen consumption, allowing oxygen ingression with time.
Uchida, Shunsuke; Hata, Kuniki; Hanawa, Satoshi
JAEA-Data/Code 2024-003, 119 Pages, 2025/01
The calculation code for determining corrosive circumstance in light water reactors, WRAC-JAEA, has been developed based on water radiolysis calculation codes for BWR. The code has involved several new calculation functions to apply it for PWR, i.e., (1) high temperature pH (pH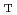 ), (2) pH
), (2) pH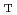 effects on water radiolysis, (3) electrochemical corrosion potential (ECP) based on the mixed potential theory, and (4) ECP based on the water radiolysis calculation results. Moderation of corrosive conditions in the primary cooling systems is one of the promising procedures to mitigate the loss of reliabilities of major components in the systems, especially in aging plants. However, water chemistry control for corrosive environment mitigation procedures are much different in BWRs and PWRs. In BWRs, intergranular stress corrosion cracking (IGSCC) of stainless steel is the dominant causes for determining plant reliability. It is difficult to increase pH and injected hydrogen amounts due to direct power cycle operation. So, precise control of hydrogen injection with supported by water radiolysis and ECP analyses has been carried out to keep material reliability. In PWRs, it is possible to maintain stable control of corrosive circumstances with higher pH and sufficiently large hydrogen concentration. Recently, it was pointed out that one of the major causes of primary water stress corrosion cracking (PWSCC) of nickel alloys was hydrogen. The optimal hydrogen concentration should be evaluated to mitigate ECP without increasing hydrogen concentration. For this, a combined water radiolysis and ECP analysis code is required to determine the suitable hydrogen concentration and ECP. WRAC-JAEA can contribute not only to evaluation of corrosive conditions each of BWR and PWR, but also to prepare for suitable countermeasures for both BWR and PWR by cross-talking the knowledge and experience with assistance of the code results.
effects on water radiolysis, (3) electrochemical corrosion potential (ECP) based on the mixed potential theory, and (4) ECP based on the water radiolysis calculation results. Moderation of corrosive conditions in the primary cooling systems is one of the promising procedures to mitigate the loss of reliabilities of major components in the systems, especially in aging plants. However, water chemistry control for corrosive environment mitigation procedures are much different in BWRs and PWRs. In BWRs, intergranular stress corrosion cracking (IGSCC) of stainless steel is the dominant causes for determining plant reliability. It is difficult to increase pH and injected hydrogen amounts due to direct power cycle operation. So, precise control of hydrogen injection with supported by water radiolysis and ECP analyses has been carried out to keep material reliability. In PWRs, it is possible to maintain stable control of corrosive circumstances with higher pH and sufficiently large hydrogen concentration. Recently, it was pointed out that one of the major causes of primary water stress corrosion cracking (PWSCC) of nickel alloys was hydrogen. The optimal hydrogen concentration should be evaluated to mitigate ECP without increasing hydrogen concentration. For this, a combined water radiolysis and ECP analysis code is required to determine the suitable hydrogen concentration and ECP. WRAC-JAEA can contribute not only to evaluation of corrosive conditions each of BWR and PWR, but also to prepare for suitable countermeasures for both BWR and PWR by cross-talking the knowledge and experience with assistance of the code results.
Imagawa, Yuya; Toyota, Kodai; Onizawa, Takashi; Kato, Shoichi
JAEA-Data/Code 2024-010, 90 Pages, 2024/11
To establish a material testing technique in sodium and to develop a method to evaluate the sodium environmental effects, sodium tests on fast reactor fuel cladding have been carried out. Fast reactor fuel cladding is susceptible to corrosion thinning and compositional change due to sodium because of its high temperature (around 675 C) and thin wall (about 0.5 mm) during normal operation. Therefore, it is important to evaluate the corrosion behavior and mechanical properties under a high-temperature sodium environment. This report summarizes the results of experimental studies on corrosion behavior and mechanical properties of modified type 316 stainless steel fuel cladding applied to actual fast reactors under a high-temperature sodium environment, in order to reflect the results to future research activities and to consolidate knowledge and experience.
C) and thin wall (about 0.5 mm) during normal operation. Therefore, it is important to evaluate the corrosion behavior and mechanical properties under a high-temperature sodium environment. This report summarizes the results of experimental studies on corrosion behavior and mechanical properties of modified type 316 stainless steel fuel cladding applied to actual fast reactors under a high-temperature sodium environment, in order to reflect the results to future research activities and to consolidate knowledge and experience.
Soma, Yasutaka; Komatsu, Atsushi; Igarashi, Takahiro
Dai-71-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.253 - 256, 2024/11
In our previous study, we reported that Cl ions penetrating stainless steel crevices do not dissipate by diffusion, even in high-purity water (i.e., conductivity remains stable), likely due to electrochemical reactions inside and outside the crevice. This study further analyzes ion behavior by experimentally and computationally investigating ion concentration drivers in high-purity water. Results show that, at 50 C, the crevice conductivity of SUS316L stainless steel reached 100
C, the crevice conductivity of SUS316L stainless steel reached 100  S/cm (100-1000 times bulk water). Modeling suggests this is due to metal cations and hydroxide ions from dissolved oxygen reduction. The dissolution rate was estimated at 10nA/cm
S/cm (100-1000 times bulk water). Modeling suggests this is due to metal cations and hydroxide ions from dissolved oxygen reduction. The dissolution rate was estimated at 10nA/cm .
.
Sato, Nobuaki*; Kameo, Yutaka; Sato, Soichi; Kumagai, Yuta; Sato, Tomonori; Yamamoto, Masahiro*; Watanabe, Yutaka*; Nagai, Takayuki; Niibori, Yuichi*; Watanabe, Masayuki; et al.
Introduction to Dismantling and Decommissioning Chemistry, 251 Pages, 2024/09
This book focuses on the dismantling and decommissioning of nuclear facilities and reactors that have suffered severe accidents. In Part 1, we introduce basic aspects ranging from fuel chemistry, analytical chemistry, radiation chemistry, corrosion, and decontamination chemistry to waste treatment and disposal. Then, Part 2 covers the chemistry involved in the decommissioning of various nuclear facilities, and discusses what chemical approaches are necessary and possible for the decommissioning of TEPCO's Fukushima Daiichi Nuclear Power Plants, how decommissioning should be carried out, and what kind of research and development and also human resource development are required for this.
Irisawa, Eriko
Taikabutsu, 76(8), p.326 - 332, 2024/08
Liquid lead-bismuth eutectic (LBE) alloys have been considered as coolants for nuclear reactors, and research and development for LBE reactors has been conducted for many years. One of the most serious problems in using LBE as a coolant is the corrosion degradation of metallic structural materials due to exposure to high operating temperatures and flowing LBE. In the case of austenitic steels containing metal components such as Ni that are easily dissolved in LBE, it is known that it is necessary to monitor and control the concentration of dissolved oxygen in LBE in order to evaluate the amount of corrosion. This paper focuses on the relationship between the dissolved oxygen concentration, which is an important factor in the corrosion degradation mechanism, and the corrosion evaluation of stainless steel in LBE. The relationship between dissolved oxygen concentration in LBE, corrosion behavior and corrosion rate will be introduced using the example of corrosion evaluation of austenitic stainless steels in LBE in this paper.
Sato, Tomonori; Hata, Kuniki; Kato, Chiaki; Igarashi, Takahiro
Zairyo To Kankyo, 73(4), p.102 - 109, 2024/04
To evaluate the effects of dissolved oxygen concentration to water quality within SCC crack and the distribution of water quality in the depth direction under irradiation, immersion tests of stainless steel specimens given a gap and water radiolysis calculations for the water quality in the crevice gap were performed. As a result, it was confirmed that Fe O
O was formed in the entire area within the crevice regardless of the dissolved oxygen concentration. It was also estimated that under irradiation, the oxidant species produced directly by radiolysis in the crack are consumed by the oxide growth, and anion enrichment occurs in the crack even in the irradiation conditions.
was formed in the entire area within the crevice regardless of the dissolved oxygen concentration. It was also estimated that under irradiation, the oxidant species produced directly by radiolysis in the crack are consumed by the oxide growth, and anion enrichment occurs in the crack even in the irradiation conditions.
Tang, J.*; Wang, Y.*; Fujihara, Hiro*; Shimizu, Kazuyuki*; Hirayama, Kyosuke*; Ebihara, Kenichi; Takeuchi, Akihisa*; Uesugi, Masayuki*; Toda, Hiroyuki*
Scripta Materialia, 239, p.115804_1 - 115804_5, 2024/01
Times Cited Count:11 Percentile:82.25(Nanoscience & Nanotechnology)Stress corrosion cracking (SCC) behaviors induced by the combination of external and internal hydrogen (H) in an Al-Zn-Mg-Cu alloy were systematically investigated via in situ 3D characterization techniques. SCC of the Al-Zn-Mg-Cu alloy could initiate and propagate in the potential crack region where the H concentration exceeded a critical value, in which the nanoscopic H-induced decohesion of 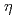 -MgZn
-MgZn precipitates resulted in macroscopic cracking. External H that penetrated the alloy from the environment played a crucial role during the SCC of the Al-Zn-Mg-Cu alloy by generating gradient-distributed H-affected zones near the crack tips, which made Al alloys in water environment more sensitive to SCC. Additionally, the pre-existing internal H was driven toward the crack tips during plastic deformation. It was involved in the SCC and made contributions to both the cracks initiation and propagation.
precipitates resulted in macroscopic cracking. External H that penetrated the alloy from the environment played a crucial role during the SCC of the Al-Zn-Mg-Cu alloy by generating gradient-distributed H-affected zones near the crack tips, which made Al alloys in water environment more sensitive to SCC. Additionally, the pre-existing internal H was driven toward the crack tips during plastic deformation. It was involved in the SCC and made contributions to both the cracks initiation and propagation.
 Sr and
Sr and  Cs in HCl solutions on the corrosion potential of Type 316L stainless steel
Cs in HCl solutions on the corrosion potential of Type 316L stainless steelAoyama, Takahito; Sato, Tomonori; Ueno, Fumiyoshi; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Igarashi, Takahiro
Zairyo To Kankyo, 72(11), p.284 - 288, 2023/11
no abstracts in English
Soma, Yasutaka; Igarashi, Takahiro
Dai-70-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.199 - 202, 2023/10
Since an acidic corrosive environment (crevice environment) is formed inside the stress corrosion cracking (SCC) of stainless steel in high temperature water, it is important to understand the corrosion behavior in the crevice environment for the better understanding of crack growth behavior. In the previous study, the authors measured the electrical conductivity inside the crevice and obtained values of 380  S/cm and 1600
S/cm and 1600  S/cm for the crevice with and without intergranular corrosion, respectively. In this study, we defined the crevice environment I (pH
S/cm for the crevice with and without intergranular corrosion, respectively. In this study, we defined the crevice environment I (pH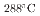 =4.41) and II (pH
=4.41) and II (pH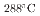 =3.13) corresponding the above conductivity values, and the corrosion behavior of Fe-xCr-20Ni (x=16.9, 19.8, 22.9, 24.3, 25.9) in each crevice environment was investigated. In the simulated crevice environment-I, the all alloys showed passive behavior, while in the environment-II, severe corrosion with intergranular cracking was observed for x = 16.9 and 19.8, and a thick oxide film was formed. On the other hand, above x=22.9, oxide film growth was suppressed and a clear passive region appeared on the polarization curve.
=3.13) corresponding the above conductivity values, and the corrosion behavior of Fe-xCr-20Ni (x=16.9, 19.8, 22.9, 24.3, 25.9) in each crevice environment was investigated. In the simulated crevice environment-I, the all alloys showed passive behavior, while in the environment-II, severe corrosion with intergranular cracking was observed for x = 16.9 and 19.8, and a thick oxide film was formed. On the other hand, above x=22.9, oxide film growth was suppressed and a clear passive region appeared on the polarization curve.
Onuki, Toshihiko*; Nakase, Masahiko*; Liu, J.; Dotsuta, Yuma; Satou, Yukihiko; Kitagaki, Toru; Kozai, Naofumi
Journal of Nuclear Science and Technology, 61(3), p.384 - 396, 2023/07
Times Cited Count:5 Percentile:73.39(Nuclear Science & Technology)Ioka, Ikuo; Kuriki, Yoshiro*; Iwatsuki, Jin; Kubo, Shinji; Yokota, Hiroki*; Kawai, Daisuke*
Proceedings of 30th International Conference on Nuclear Engineering (ICONE30) (Internet), 5 Pages, 2023/05
A thermochemical water-splitting iodine-sulfur processes (IS process) is one of candidates for the large-scale production of hydrogen using heat from solar power. Severe corrosive environment which is thermal decomposition of sulfuric acid exists in the IS process. A hybrid material with the corrosion-resistance and the ductility was made by a plasma spraying and laser treatment. The specimen had excellent corrosion resistance in the condition of 95 mass% boiling sulfuric acid. This was attributed to the formation of SiO on the surface. A corrosion test of the container was performed in 95 mass% boiling sulfuric acid until 500 hours to evaluate the corrosion characteristic of the container. No peeling was observed on the inner surface of the container after the corrosion test. The container had excellent corrosion resistance in the condition of 95 mass% boiling sulfuric acid.
on the surface. A corrosion test of the container was performed in 95 mass% boiling sulfuric acid until 500 hours to evaluate the corrosion characteristic of the container. No peeling was observed on the inner surface of the container after the corrosion test. The container had excellent corrosion resistance in the condition of 95 mass% boiling sulfuric acid.
Hata, Kuniki; Kimura, Atsushi*; Taguchi, Mitsumasa*; Sato, Tomonori; Kato, Chiaki; Watanabe, Yutaka*
Zairyo To Kankyo, 72(4), p.126 - 130, 2023/04
Gamma-radiolysis experiments with gas-liquid coexistent samples were carried out to investigate effects of gas-phase radiolysis on corrosive environment for materials in solutions under irradiation. After gamma-ray irradiation, hydrogen peroxide, nitrate ion, nitrite ion were detected in the liquid phase. The production yields of nitrate ion and nitrite ion increased with increasing gas-phase volume and oxygen concentration. This result indicated that chemical reactions including oxygen and nitrogen in the gas phase were required for the production of nitrate ion and nitrite ion. To magnify the effects of gas-phase radiolysis in the gas-liquid coexistent samples, absorption dose rate in the liquid phase was reduced by one-hundredth using lead shield. The concentration of hydrogen peroxide and the pH in the shielded liquid phase were similar to those in the irradiated pure water, which did not contact with gas phase. This result indicated that the effects of nitrate ion and nitrite ion dissolved in the liquid phase on water radiolysis were not important in the current experimental system, in which the effects of gas-phase radiolysis were increased by 100-times.
Ao, N.*; Zhang, H.*; Xu, H. H.*; Wu, S. C.*; Liu, D.*; Xu, P. G.; Su, Y. H.; Kang, Q. H.*; Kang, G. Z.*
Engineering Fracture Mechanics, 281, p.109166_1 - 109166_14, 2023/03
Times Cited Count:12 Percentile:83.88(Mechanics)Haoran, W.*; Yu, H.*; Liu, J.*; Kondo, Sosuke*; Okubo, Nariaki; Kasada, Ryuta*
Corrosion Science, 209, p.110818_1 - 110818_12, 2022/12
Times Cited Count:16 Percentile:79.63(Materials Science, Multidisciplinary)The corrosion behavior of newly developed Al O
O forming high Mn oxide dispersion strengthened (ODS) austenitic steels was examined in oxygen-saturated lead-bismuth eutectic at 450
forming high Mn oxide dispersion strengthened (ODS) austenitic steels was examined in oxygen-saturated lead-bismuth eutectic at 450 C for 430 h. Compared with non-ODS steels, the ODS steels possessed superior resistance to corrosion and spallation. The high density grain boundaries in the ODS steels acted as channels for the rapid outward diffusion of metallic elements, forming an internal continuous Cr
C for 430 h. Compared with non-ODS steels, the ODS steels possessed superior resistance to corrosion and spallation. The high density grain boundaries in the ODS steels acted as channels for the rapid outward diffusion of metallic elements, forming an internal continuous Cr O
O scale at the original surface. Accelerated Al diffusion, along with oxidation prevention by the external (Fe, Mn) oxide scale and the internal Cr
scale at the original surface. Accelerated Al diffusion, along with oxidation prevention by the external (Fe, Mn) oxide scale and the internal Cr O
O scale, jointly resulted in the formation of a continuous Al-rich oxide scale in ODS-7Al steel, contributing to its superior corrosion resistance.
scale, jointly resulted in the formation of a continuous Al-rich oxide scale in ODS-7Al steel, contributing to its superior corrosion resistance.
Hata, Kuniki; Sato, Tomonori
Hoshasen Kagaku (Internet), (114), p.33 - 38, 2022/10
no abstracts in English
Sato, Tomonori; Hata, Kuniki; Kaji, Yoshiyuki; Taguchi, Mitsumasa*; Seito, Hajime*; Inoue, Hiroyuki*; Tada, Eiji*; Abe, Hiroshi*; Akiyama, Eiji*; Suzuki, Shunichi*
Isotope News, (782), p.40 - 44, 2022/08
The stagnant water in the reactor building at Fukushima Daiichi Nuclear Power Station (1F) is exposed to the radiation from fuel debris and radioactive species. This water contains much amounts of impurities from the seawater which was injected in the emergency cooling. The impurities will affect the radiolysis and corrosive conditions in the water under irradiation. So, the water radiolysis data, corrosion data of steels under irradiations, and the evaluated potential impacts of corrosion in the decommissioning process of 1F are arranged as the database for corrosion under irradiation. This paper introduces the outline of this database.
Yamaguchi, Yoshihito; Mano, Akihiro; Li, Y.
Proceedings of ASME 2022 Pressure Vessels and Piping Conference (PVP 2022) (Internet), 10 Pages, 2022/07
The steam generator (SG) tube is one of the important components in pressurized water reactors. Flaws such as wall-thinning or stress corrosion cracking have been reported in SG tubes. The burst pressure where both the internal and external pressures from the primary and secondary coolant systems are considered must be predicted to assess the structural integrity of SG tubes. Burst tests were performed by various organizations. On the basis of the test results, failure estimation methods were proposed. In this study, previous burst test data and existing failure estimation methods for SG tubes with wall-thinning or crack were investigated. As a result, the coefficient of the existing estimation method for SG tube with uniform wall-thinning was updated. In addition, failure estimation methods that are suitable for SG tubes with crack or local wall-thinning were proposed by considering the effects of the flaw shape and size on the burst pressure. The applicability of the failure estimation methods was confirmed by comparing the predicted results with the burst test data in actual SG tubes.
Soma, Yasutaka; Komatsu, Atsushi; Ueno, Fumiyoshi
Corrosion, 78(6), p.503 - 515, 2022/06
Times Cited Count:1 Percentile:7.57(Materials Science, Multidisciplinary)The effects of electrochemical potential (ECP) on water chemistry within a crevice are of critical importance for understanding stress corrosion cracking (SCC) of Fe-Cr-Ni alloys in high temperature water. In this study, the effects of ECP on the electrical conductivity of a solution within a Type-316L stainless steel crevice (
 ) have been studied in 288
) have been studied in 288 C and 8 MPa water containing 10 ppb Cl
C and 8 MPa water containing 10 ppb Cl as major anionic species. In situ measurements of
as major anionic species. In situ measurements of 
 in a rectangular crevice with a gap of 15
in a rectangular crevice with a gap of 15  m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in
m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in 
 from 12
from 12  Scm
Scm to 160
to 160  Scm
Scm at a distance of 21 mm from the crevice mouth. The increase in
at a distance of 21 mm from the crevice mouth. The increase in 
 reached a maximum at about 0.15 V (about 300
reached a maximum at about 0.15 V (about 300  Scm
Scm ) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl
) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl is the major anionic species transported into the crevice at relatively low potentials, and that
is the major anionic species transported into the crevice at relatively low potentials, and that 
 increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
 generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(5), p.133 - 137, 2022/05
The effect of the corrosion inhibitor on the corrosion of steel under a thin solution layer was investigated. As a result of forming a thin solution layer with a thickness of 1.0-0.2 mm on the specimen, adding a mixed solution of sodium molybdate and aluminum lactate as a corrosion inhibitor, and performing electrochemical measurement, the corrosion inhibitor suppresses the anodic reaction. And in the thin solution layer, it was suggested that the morphology of the protective layer structure by the corrosion inhibitor changed according to the amount of liquid as compared with the bulk immersion.