Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
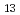 } polyoxo cluster U
} polyoxo cluster U O
O Cl
Cl (MeO)
(MeO)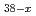 (x = 2.3, MeO = methoxide)
(x = 2.3, MeO = methoxide)Fichter, S.*; Radoske, T.*; 池田 篤史
Acta Crystallographica Section E; Crystallographic Communications (Internet), 77(8), p.847 - 852, 2021/08
A new type of polyoxo cluster complex that contains thirteen uranium atoms, {U }, was synthesised and characterised as [U
}, was synthesised and characterised as [U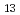 (
(
 -O
-O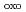 )
) (
(
 -O
-O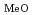 )
) (
(
 -O
-O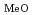 )
)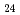 Cl
Cl (O
(O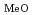 )
)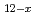 ] (x = 2.3, MeO = methoxide) (I). The complex crystallises from methanol containing tetravalent uranium (U
] (x = 2.3, MeO = methoxide) (I). The complex crystallises from methanol containing tetravalent uranium (U ) with a basic organic ligand. The characterised {U
) with a basic organic ligand. The characterised {U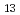 } polyoxo cluster complex possesses a single cubic uranium polyhedron at the centre of the cluster core. The observed shortening of U-O bonds, together with BVS calculations and the overall negative charge (2-) of (I), suggests that the central uranium atom in (I), that forms the single cubic polyhedron, is presumably oxidised to the pentavalent state (U
} polyoxo cluster complex possesses a single cubic uranium polyhedron at the centre of the cluster core. The observed shortening of U-O bonds, together with BVS calculations and the overall negative charge (2-) of (I), suggests that the central uranium atom in (I), that forms the single cubic polyhedron, is presumably oxidised to the pentavalent state (U ) from the original tetravalent state (U
) from the original tetravalent state (U ). Complex I is, hence, the first example of a polyoxo cluster possessing a single cubic coordination polyhedron of U
). Complex I is, hence, the first example of a polyoxo cluster possessing a single cubic coordination polyhedron of U .
.
 O
O -type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solution
-type Schiff base complexes of tetravalent f-elements (M(IV) = Ce, Th, U, Np, and Pu) in solid state and in solutionRadoske, T.*; Kloditz, R.*; Fichter, S.*; M rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; 池田 篤史
rz, J.*; Kaden, P.*; Patzschke, M.*; Schmidt, M.*; Stumpf, T.*; Walter, O.*; 池田 篤史
Dalton Transactions (Internet), 49(48), p.17559 - 17570, 2020/12
被引用回数:17 パーセンタイル:74.45(Chemistry, Inorganic & Nuclear)A series of tetradentate N O
O -type Schiff base complexes with tetravalent 4f- and 5f-block metals, [M(salpn)
-type Schiff base complexes with tetravalent 4f- and 5f-block metals, [M(salpn) ] (H
] (H salpn =
salpn = 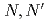 -disalicylidene-1,3-diaminopropane; M = Ce, Th, U, Np, and Pu), were prepared to systematically investigate their solid state structure, and their complexation behaviour in solution with the goal to investigate the subtle differences between 4f- and 5f-elements. X-ray diffraction revealed that all investigated metal cations form [M(salpn)
-disalicylidene-1,3-diaminopropane; M = Ce, Th, U, Np, and Pu), were prepared to systematically investigate their solid state structure, and their complexation behaviour in solution with the goal to investigate the subtle differences between 4f- and 5f-elements. X-ray diffraction revealed that all investigated metal cations form [M(salpn) ] complexes. All the complexes show the same ligand arrangement with meridional conformation, amongst which only Ce(IV) exhibits unique behaviour upon crystallisation. [Ce(salpn)
] complexes. All the complexes show the same ligand arrangement with meridional conformation, amongst which only Ce(IV) exhibits unique behaviour upon crystallisation. [Ce(salpn) ] crystallises in two less symmetric systems (
] crystallises in two less symmetric systems (
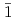 or
or  2
2 /
/ ), whilst all the other [M(salpn)
), whilst all the other [M(salpn) ] crystallise in a more symmetric orthorhombic system (
] crystallise in a more symmetric orthorhombic system ( ban). Quantum chemical calculations suggest that the observed structural peculiarity of Ce(IV) stems from the geometrical flexibility due to the more "ionic" nature of bonds to the 4f element.
ban). Quantum chemical calculations suggest that the observed structural peculiarity of Ce(IV) stems from the geometrical flexibility due to the more "ionic" nature of bonds to the 4f element.  H NMR measurements revealed that [M(salpn)
H NMR measurements revealed that [M(salpn) ] forms two different species in solution with and without an additional solvent molecule, where the relative distribution of the two species depends mainly on the ionic radius of the metal centre. Again, Ce(IV) behaves differently from the tetravalent actinides with a higher ratio of the solvent-molecule-coordinated species than the ratio expected from its ionic radius. Hence, this study is successful in observing subtle differences between 4f- (
] forms two different species in solution with and without an additional solvent molecule, where the relative distribution of the two species depends mainly on the ionic radius of the metal centre. Again, Ce(IV) behaves differently from the tetravalent actinides with a higher ratio of the solvent-molecule-coordinated species than the ratio expected from its ionic radius. Hence, this study is successful in observing subtle differences between 4f- (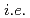 Ce) and 5f-elements (actinides; Th, U, Np, and Pu) both in the solid state and in solution on an analytically distinguishable level, and in relating the observed subtle differences to their electronic structure.
Ce) and 5f-elements (actinides; Th, U, Np, and Pu) both in the solid state and in solution on an analytically distinguishable level, and in relating the observed subtle differences to their electronic structure.