Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 and their Zr-O surface modified electrodes
and their Zr-O surface modified electrodesAbe, Machiko*; Iba, Hideki*; Suzuki, Kota*; Minamishima, Hiroaki*; Hirayama, Masaaki*; Tamura, Kazuhisa; Mizuki, Junichiro*; Saito, Tomohiro*; Ikuhara, Yuichi*; Kanno, Ryoji*
Journal of Power Sources, 345, p.108 - 119, 2017/03
Times Cited Count:12 Percentile:37.12(Chemistry, Physical)The surface structure of the Li(Ni, Co, Mn)O electrode was studied during charge/discharge process using electrochemical methods and X-ray/Neutron scattering techniques. It was found that during charge/discharge process the coverage of spinel structure increased. The spinel structure has low electrochemical activity and is not involved in Li insertion/extraction. After the surface modification, it was found that the coverage of the spinel structure did not increase. Further, it was also found out that the Li concentration at the electrode/electrolyte interface increased.
electrode was studied during charge/discharge process using electrochemical methods and X-ray/Neutron scattering techniques. It was found that during charge/discharge process the coverage of spinel structure increased. The spinel structure has low electrochemical activity and is not involved in Li insertion/extraction. After the surface modification, it was found that the coverage of the spinel structure did not increase. Further, it was also found out that the Li concentration at the electrode/electrolyte interface increased.
Makabe, Takeshi*; Prawitwong, P.*; Takahashi, Ryo*; Takigami, Machiko*; Nagasawa, Naotsugu; Takigami, Shoji*
Transactions of the Materials Research Society of Japan, 33(2), p.471 - 474, 2008/06
Konjac mannan (KM) is a water soluble glucomannan with high molar mass. KM aqueous solution shows extremely high viscosity. The effects of  -rays irradiation and acid hydrolysis on molar mass were studied. The hydrolysis was carried out using citric acid. Characteristics of the irradiated and hydrolyzed KM were investigated using GPC-MALLS and a viscometer. The chemical structure of KM scarcely changed by both treatments. Molar mass of the irradiated KM decreased gradually with increasing dose. Molar mass of the hydrolyzed KM also decreased gradually with acid concentration. The viscosity of both treated KM aqueous solutions decreased with decreasing molar mass. High molar mass KM solution showed pseudo-plastic fluids behavior of the non-Newtonian fluid at dilute region and changed to Newtonian fluid with decrease of molar mass. Low molar mass KM solution showed behavior of Newtonian fluid at semi-dilute region. The critical concentration at the overlap limit of KM solution increased with decreasing of molar mass.
-rays irradiation and acid hydrolysis on molar mass were studied. The hydrolysis was carried out using citric acid. Characteristics of the irradiated and hydrolyzed KM were investigated using GPC-MALLS and a viscometer. The chemical structure of KM scarcely changed by both treatments. Molar mass of the irradiated KM decreased gradually with increasing dose. Molar mass of the hydrolyzed KM also decreased gradually with acid concentration. The viscosity of both treated KM aqueous solutions decreased with decreasing molar mass. High molar mass KM solution showed pseudo-plastic fluids behavior of the non-Newtonian fluid at dilute region and changed to Newtonian fluid with decrease of molar mass. Low molar mass KM solution showed behavior of Newtonian fluid at semi-dilute region. The critical concentration at the overlap limit of KM solution increased with decreasing of molar mass.
 synchrotron X-ray reflectometry; A New experimental technique for LiCoO
synchrotron X-ray reflectometry; A New experimental technique for LiCoO model electrode
model electrodeHirayama, Masaaki*; Sonoyama, Noriyuki*; Abe, Takashi*; Minoura, Machiko*; Ito, Masumi*; Mori, Daisuke*; Yamada, Atsuo*; Kanno, Ryoji*; Terashima, Takahito*; Takano, Mikio*; et al.
Journal of Power Sources, 168(2), p.493 - 500, 2007/06
Times Cited Count:93 Percentile:90.14(Chemistry, Physical)A new experimental technique was developed for detecting structure changes at electrode/electrolyte interface of lithium cell using X-ray reflectometry and two-dimensional model electrodes with a restricted lattice-plane. The electrodes were constructed with an epitaxial film of LiCoO synthesized by pulsed laser deposition method. The anisotropic properties were confirmed by electrochemical measurements.
synthesized by pulsed laser deposition method. The anisotropic properties were confirmed by electrochemical measurements. 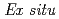 X-ray reflectivity measurements indicated that the impurity layer existed on the as-grown LiCoO
X-ray reflectivity measurements indicated that the impurity layer existed on the as-grown LiCoO was dissolved and a new SEI layer with lower density was formed after soaking into the electrolyte.
was dissolved and a new SEI layer with lower density was formed after soaking into the electrolyte.  X-ray reflectivity measurements indicated that the surface roughness of the intercalation (1 1 0) plane increased with applying voltages, while no significant changes in surface morphology were observed for the intercalation non-active (0 0 3) plane during the pristine stage of the charge-discharge process.
X-ray reflectivity measurements indicated that the surface roughness of the intercalation (1 1 0) plane increased with applying voltages, while no significant changes in surface morphology were observed for the intercalation non-active (0 0 3) plane during the pristine stage of the charge-discharge process.
 -ray irradiation
-ray irradiationAbe, Yasuhiro*; Takigami, Machiko; Sugino, Koji*; Taguchi, Mitsumasa; Kojima, Takuji; Umemura, Tomonari*; Tsunoda, Kinichi*
Bulletin of the Chemical Society of Japan, 76(8), p.1681 - 1685, 2003/08
Times Cited Count:5 Percentile:26.19(Chemistry, Multidisciplinary)The decomposition of phenolic endocrine disrupting chemicals (P-EDCs), such as phenol, 4-butylphenol (BuP), and bisphenol A (BPA), in aqueous solutions by potassium permanganate (KMnO ) was studied and its efficiency was compared with that of hydroxyl radicals (OH
) was studied and its efficiency was compared with that of hydroxyl radicals (OH ) generated by
) generated by  Co
Co  -ray irradiation. Various organic acids and inorganic carbon were formed in the decomposition of P-EDCs due to either KMnO
-ray irradiation. Various organic acids and inorganic carbon were formed in the decomposition of P-EDCs due to either KMnO or OH
or OH . They were formed via direct aromatic ring cleavage in the case of KMnO
. They were formed via direct aromatic ring cleavage in the case of KMnO and OH
and OH addition-substitution reactions followed by aromatic ring cleavage in the case of OH
addition-substitution reactions followed by aromatic ring cleavage in the case of OH . Comparing the decrease in the P-EDCs based on the number of electrons, the amount of KMnO
. Comparing the decrease in the P-EDCs based on the number of electrons, the amount of KMnO spent to completely eliminate BuP and BPA was comparable to the amount of OH
spent to completely eliminate BuP and BPA was comparable to the amount of OH . Although three times more KMnO
. Although three times more KMnO was needed for phenol than OH
was needed for phenol than OH , the complete conversion of phenol into organic acids and inorganic carbon was achieved with 720
, the complete conversion of phenol into organic acids and inorganic carbon was achieved with 720 M of electrons in both cases.
M of electrons in both cases.