Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ito, Kotaro*; Tamura, Kazuhisa; Shimizu, Keisuke*; Yamada, Norifumi*; Watanabe, Kenta*; Suzuki, Kota*; Kanno, Ryoji*; Hirayama, Masaaki*
RSC Applied Interfaces (Internet), 1(4), p.790 - 799, 2024/04
LiCoO is widely used as a cathode material in lithium-ion batteries. However, the reversible capacity of LiCoO
is widely used as a cathode material in lithium-ion batteries. However, the reversible capacity of LiCoO at high voltage is not well known because of the oxidative degradation of the electrolyte. In this study, a thin-film all-solid-state battery was fabricated with epitaxially grown LiCoO
at high voltage is not well known because of the oxidative degradation of the electrolyte. In this study, a thin-film all-solid-state battery was fabricated with epitaxially grown LiCoO cathode and Li
cathode and Li PO
PO solid electrolyte as a model battery that operates stably at high voltages, ranging up to 4.6 V, without drastic degradation. However, the charge-discharge capacities of the battery decreased with cycling at 4.7 V.
solid electrolyte as a model battery that operates stably at high voltages, ranging up to 4.6 V, without drastic degradation. However, the charge-discharge capacities of the battery decreased with cycling at 4.7 V.  synchrotron X-ray diffraction studies revealed that LiCoO
synchrotron X-ray diffraction studies revealed that LiCoO was deactivated via a change in its crystal structure to O1 type, with narrow interlayer distances, at 4.7 V. The reduced distance between the interlayers in the O1 structure possibly prevents the re-intercalation of Li ions, leading to irreversibility.
was deactivated via a change in its crystal structure to O1 type, with narrow interlayer distances, at 4.7 V. The reduced distance between the interlayers in the O1 structure possibly prevents the re-intercalation of Li ions, leading to irreversibility.
Yoshimoto, Masataka*; Tamura, Kazuhisa; Watanabe, Kenta*; Shimizu, Keisuke*; Horisawa, Yuhei*; Kobayashi, Takeshi*; Tsurita, Hanae*; Suzuki, Kota*; Kanno, Ryoji*; Hirayama, Masaaki*
Sustainable Energy & Fuels (Internet), 8(6), p.1236 - 1244, 2024/03
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)Photo-rechargeable systems, which can efficiently convert and store solar energy into chemical energy within single devices, are essential to harness sunlight effectively. Photo-(de)intercalation plays a pivotal role in the functionality of photorechargeable systems. Nevertheless, the photo-(de)intercalation process has not been conclusively confirmed owing to potential interference from side reactions, such as the decomposition of liquid electrolytes and the elution of electrode materials. In this study, we successfully demonstrated photo-responsive Li -deintercalation using an all-solid-state thin-film battery comprised of epitaxially-grown anatase TiO
-deintercalation using an all-solid-state thin-film battery comprised of epitaxially-grown anatase TiO doped with Nb (a-TiO
doped with Nb (a-TiO :Nb) as the cathode. Under light irradiation, Li
:Nb) as the cathode. Under light irradiation, Li -deintercalation occurred and was subsequently reversibly intercalated into a-TiO
-deintercalation occurred and was subsequently reversibly intercalated into a-TiO :Nb during discharge.
:Nb during discharge.
Watanabe, Kenta*; Horisawa, Yuhei*; Yoshimoto, Masataka*; Tamura, Kazuhisa; Suzuki, Kota*; Kanno, Ryoji*; Hirayama, Masaaki*
Nano Letters, 24(6), p.1916 - 1922, 2024/02
Times Cited Count:3 Percentile:74.90(Chemistry, Multidisciplinary)Electrochemistry has extended from reactions at solid/liquid interfaces to those at solid/solid interfaces. In this study, we achieve the stable photoelectrochemical reaction at the semiconductor-electrode/solid-electrolyte interface in Nb-doped anatase-TiO (a-TiO
(a-TiO :Nb)/Li
:Nb)/Li PO
PO (LPO)/Li all-solid-state cell. The oxidative currents of a-TiO
(LPO)/Li all-solid-state cell. The oxidative currents of a-TiO :Nb/LPO/Li increase upon light irradiation when a-TiO
:Nb/LPO/Li increase upon light irradiation when a-TiO :Nb is located at a potential that is more positive than its flat-band potential. The photoelectrochemical reaction at the semiconductor/solid-electrolyte interface is driven by the same principle as that at semiconductor/liquid-electrolyte interfaces. Thus, we extend photoelectrochemistry to all-solid-state systems composed of solid/solid interfaces.
:Nb is located at a potential that is more positive than its flat-band potential. The photoelectrochemical reaction at the semiconductor/solid-electrolyte interface is driven by the same principle as that at semiconductor/liquid-electrolyte interfaces. Thus, we extend photoelectrochemistry to all-solid-state systems composed of solid/solid interfaces.
Kusaka, Ryoji; Kumagai, Yuta; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 60(5), p.603 - 613, 2023/05
Times Cited Count:5 Percentile:61.74(Nuclear Science & Technology) , Zr, and stainless steel and leaching behavior of the fission products and matrix elements
, Zr, and stainless steel and leaching behavior of the fission products and matrix elementsTonna, Ryutaro*; Sasaki, Takayuki*; Kodama, Yuji*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta; Kusaka, Ryoji; Watanabe, Masayuki
Nuclear Engineering and Technology, 55(4), p.1300 - 1309, 2023/04
Times Cited Count:6 Percentile:80.64(Nuclear Science & Technology)Simulated debris was synthesized using UO , Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO
, Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO , whereas a (U,Zr)O
, whereas a (U,Zr)O solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U
solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U O
O and (Fe,Cr)UO
and (Fe,Cr)UO phases formed at 1473 K whereas a (U,Zr)O
phases formed at 1473 K whereas a (U,Zr)O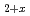 solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
Hirata, Sakiko*; Kusaka, Ryoji; Meiji, Shogo*; Tamekuni, Seita*; Okudera, Kosuke*; Hamada, Shoken*; Sakamoto, Chihiro*; Honda, Takumi*; Matsushita, Kosuke*; Muramatsu, Satoru*; et al.
Inorganic Chemistry, 62(1), p.474 - 486, 2023/01
Times Cited Count:3 Percentile:28.83(Chemistry, Inorganic & Nuclear) and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:4 Percentile:53.26(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe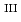 with increasing electron densities around Fe
with increasing electron densities around Fe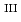 , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe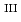
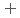 U
U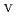
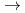 Fe
Fe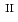
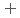 U
U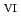 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr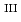 could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr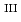 in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
 , Zr, and stainless-steel
, Zr, and stainless-steelKirishima, Akira*; Akiyama, Daisuke*; Kumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Sasaki, Takayuki*; Sato, Nobuaki*
Journal of Nuclear Materials, 567, p.153842_1 - 153842_15, 2022/08
Times Cited Count:9 Percentile:79.01(Materials Science, Multidisciplinary)To understand the chemical structure and stability of nuclear fuel debris consisting of UO , Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO
, Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO -SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600
-SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600 C, in inert (Ar) or oxidative (Ar + 2% O
C, in inert (Ar) or oxidative (Ar + 2% O ) atmospheres.
) atmospheres. 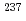 Np and
Np and 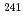 Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M
Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M ssbauer spectroscopy, which provided the major uranium phase of the UO
ssbauer spectroscopy, which provided the major uranium phase of the UO  -SUS-Zr debris was the solid solution of U
-SUS-Zr debris was the solid solution of U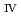 O
O (s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO
(s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO -SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
-SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
 O
O solution
solutionKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Journal of Nuclear Science and Technology, 59(8), p.961 - 971, 2022/08
Times Cited Count:3 Percentile:42.88(Nuclear Science & Technology)We investigated potential degradation of fuel debris caused by H O
O , which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H
, which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H O
O solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H
solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H O
O . Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H
. Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H O
O reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO
reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO improves the stability of fuel debris against H
improves the stability of fuel debris against H O
O reaction.
reaction.
Kusaka, Ryoji; Watanabe, Masayuki
Journal of Physical Chemistry Letters (Internet), 13(30), p.7065 - 7071, 2022/08
Times Cited Count:10 Percentile:72.53(Chemistry, Physical) O
O -induced oxidative degradation of simulated fuel debris
-induced oxidative degradation of simulated fuel debrisKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Hoshasen Kagaku (Internet), (113), p.61 - 64, 2022/04
The severe accident at TEPCO's Fukushima Daiichi Nuclear Power Station resulted in generation of fuel debris. The fuel debris is in contact with water and the radiolysis of water can accelerate degradation of the debris. The analysis of particles sampled from inside or near the damaged reactors indicates the complicated compositions of the fuel debris. It is challenging to estimate the effect of water radiolysis on such a complicated material. Therefore, in this study, we investigated the potential degradation process by leaching experiments of simulated fuel debris in aqueous H O
O solution. The results show that the reaction of H
solution. The results show that the reaction of H O
O induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H
induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H O
O . These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
. These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
 O
O ; Application of Raman imaging technique to uranium compound
; Application of Raman imaging technique to uranium compoundKusaka, Ryoji; Kumagai, Yuta; Yomogida, Takumi; Takano, Masahide; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 58(6), p.629 - 634, 2021/06
Times Cited Count:8 Percentile:60.88(Nuclear Science & Technology)Kusaka, Ryoji; Watanabe, Masayuki
Journal of Physical Chemistry B, 125(24), p.6727 - 6731, 2021/06
Times Cited Count:13 Percentile:41.61(Chemistry, Physical)Kubota, Tomohiro; Kuroda, Hisao*; Watanabe, Mirai*; Takahashi, Akiko*; Nakazato, Ryoji*; Tarui, Mika*; Matsumoto, Shunichi*; Nakagawa, Keita*; Numata, Yasuko*; Ouchi, Takao*; et al.
Atmospheric Environment, 243, p.117856_1 - 117856_9, 2020/12
Times Cited Count:4 Percentile:10.92(Environmental Sciences)The dry and wet depositions of atmospheric ammonia (NH ) is one of the important pathways of nitrogen loads to aquatic ecosystems. Crop and livestock agriculture, one of the largest emitters of NH
) is one of the important pathways of nitrogen loads to aquatic ecosystems. Crop and livestock agriculture, one of the largest emitters of NH in Asian countries, are known to cause high spatial and seasonal variation of NH
in Asian countries, are known to cause high spatial and seasonal variation of NH and influence the surrounding lake basin areas via its dry and wet deposition. However, the spatial characteristics of the NH
and influence the surrounding lake basin areas via its dry and wet deposition. However, the spatial characteristics of the NH concentration in basin scale are not completely understood for regulation in NH
concentration in basin scale are not completely understood for regulation in NH emission. Here we aim to clarify dominant factors of spatial and seasonal variations of the NH
emission. Here we aim to clarify dominant factors of spatial and seasonal variations of the NH concentration in a eutrophic lake basin surrounded by agricultural areas in Japan. Passive sampling over various land use categories in the basin was conducted at 36 sites in total from October 2018 to January 2020. Interestingly, the observed NH
concentration in a eutrophic lake basin surrounded by agricultural areas in Japan. Passive sampling over various land use categories in the basin was conducted at 36 sites in total from October 2018 to January 2020. Interestingly, the observed NH concentration near the livestock houses were higher in winter than summer, which was inconsistent with knowledge of seasonal changes of current NH
concentration near the livestock houses were higher in winter than summer, which was inconsistent with knowledge of seasonal changes of current NH emission inventory based on temperature-driven volatilization process. Comparing monthly NH
emission inventory based on temperature-driven volatilization process. Comparing monthly NH concentrations with various meteorological factors, we suggested the importance of seasonal advection of NH
concentrations with various meteorological factors, we suggested the importance of seasonal advection of NH from high emission sources to which has been rarely paid attention by the previous past studies. As for this, should be considered for lake ecosystem management since deposition of NH
from high emission sources to which has been rarely paid attention by the previous past studies. As for this, should be considered for lake ecosystem management since deposition of NH is known to be closely related to the ecological processes such as phytoplankton blooming.
is known to be closely related to the ecological processes such as phytoplankton blooming.
Kusaka, Ryoji; Watanabe, Masayuki
Journal of Nuclear Science and Technology, 57(9), p.1046 - 1050, 2020/09
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Otaka, Toshiki*; Sato, Tatsumi*; Ono, Shimpei; Nagoshi, Kohei; Abe, Ryoji*; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Nakatani, Kiyoharu*
Analytical Sciences, 35(10), p.1129 - 1133, 2019/10
Times Cited Count:9 Percentile:33.49(Chemistry, Analytical)Kusaka, Ryoji; Watanabe, Masayuki
Physical Chemistry Chemical Physics, 20(47), p.29588 - 29590, 2018/12
Times Cited Count:20 Percentile:68.11(Chemistry, Physical)Mechanistic understanding of solvent extraction of uranyl ions (UO
 ) by tributyl phosphate (TBP) will help improve the technology for the treatment and disposal of spent nuclear fuels. So far, it has been believed that uranyl ions in the aqueous phase are adsorbed to a TBP-enriched organic/aqueous interface, form complexes with TBP at the interface, and are extracted into the organic phase. Here we show that uranyl-TBP complex formation does not take place at the interface using vibrational sum frequency generation (VSFG) spectroscopy and propose an alternative extraction mechanism that uranyl nitrate, UO
) by tributyl phosphate (TBP) will help improve the technology for the treatment and disposal of spent nuclear fuels. So far, it has been believed that uranyl ions in the aqueous phase are adsorbed to a TBP-enriched organic/aqueous interface, form complexes with TBP at the interface, and are extracted into the organic phase. Here we show that uranyl-TBP complex formation does not take place at the interface using vibrational sum frequency generation (VSFG) spectroscopy and propose an alternative extraction mechanism that uranyl nitrate, UO (NO
(NO )
) , passes through the interface and forms the uranyl-TBP complex, UO
, passes through the interface and forms the uranyl-TBP complex, UO (NO
(NO )
) (TBP)
(TBP) , in the organic phase.
, in the organic phase.
Kusaka, Ryoji; Watanabe, Masayuki
Physical Chemistry Chemical Physics, 20(4), p.2809 - 2813, 2018/01
Times Cited Count:17 Percentile:59.67(Chemistry, Physical)Solvent extraction plays an integral part in the separation and purification of metals. Because extractants generally used as complexing agents for metal extractions, such as di-(2-ethylhexyl)phosphoric acid (HDEHP) for lanthanide extractions, are amphiphilic, they come to the organic/water interface, and the interface plays a crucial role as the site of the formation of metal complexes and subsequent transfer reaction to an organic phase. Despite the importance of the interface for solvent extractions, however, molecular-level structure of the interface is unclear because of experimental difficulty. Here we studied structure of a trivalent europium (Eu ) complex with HDEHP formed at HDEHP monolayer/water interface by heterodyne-detected vibrational sum frequency generation (HD-VSFG) spectroscopy. The study on the HDEHP/water interface enables us to investigate the structure of the interfacial Eu
) complex with HDEHP formed at HDEHP monolayer/water interface by heterodyne-detected vibrational sum frequency generation (HD-VSFG) spectroscopy. The study on the HDEHP/water interface enables us to investigate the structure of the interfacial Eu complex by excluding the migration of Eu
complex by excluding the migration of Eu into an organic phase after the complex formation at the interface. The interface-selective vibrational Im
into an organic phase after the complex formation at the interface. The interface-selective vibrational Im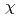
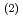 spectra observed by HD-VSFG of HDEHP/Eu(NO
spectra observed by HD-VSFG of HDEHP/Eu(NO )
) aqueous solution interface in the 2800-3500 cm
aqueous solution interface in the 2800-3500 cm region indicate that Eu
region indicate that Eu at the HDEHP/water interface is bonded by HDEHP from the air side and by water molecules from the water side. To the best of our knowledge, such metal complex structures have not been identified in the organic or water solutions.
at the HDEHP/water interface is bonded by HDEHP from the air side and by water molecules from the water side. To the best of our knowledge, such metal complex structures have not been identified in the organic or water solutions.
Nakajima, Kenji; Kawakita, Yukinobu; Ito, Shinichi*; Abe, Jun*; Aizawa, Kazuya; Aoki, Hiroyuki; Endo, Hitoshi*; Fujita, Masaki*; Funakoshi, Kenichi*; Gong, W.*; et al.
Quantum Beam Science (Internet), 1(3), p.9_1 - 9_59, 2017/12
The neutron instruments suite, installed at the spallation neutron source of the Materials and Life Science Experimental Facility (MLF) at the Japan Proton Accelerator Research Complex (J-PARC), is reviewed. MLF has 23 neutron beam ports and 21 instruments are in operation for user programs or are under commissioning. A unique and challenging instrumental suite in MLF has been realized via combination of a high-performance neutron source, optimized for neutron scattering, and unique instruments using cutting-edge technologies. All instruments are/will serve in world-leading investigations in a broad range of fields, from fundamental physics to industrial applications. In this review, overviews, characteristic features, and typical applications of the individual instruments are mentioned.
Abe, Ryoji*; Nagoshi, Kohei*; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Matsuura, Haruaki*; Takagi, Hideaki*; Shimizu, Nobutaka*; Koka, Masashi*; Sato, Takahiro*
Nuclear Instruments and Methods in Physics Research B, 404, p.173 - 178, 2017/08
Times Cited Count:7 Percentile:51.69(Instruments & Instrumentation)