Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nasu, Mitsunori*; Yanai, Hiroshi*; Hirayama, Naoki*; Adachi, Hironori*; Kakizawa, Yu*; Shirase, Yuto*; Nishiyama, Hiromichi*; Kawamoto, Teppei*; Inukai, Junji*; Shinohara, Takenao; et al.
Journal of Power Sources, 530, p.231251_1 - 231251_11, 2022/05
Times Cited Count:16 Percentile:89.77(Chemistry, Physical) Re]Organorhenium-labeled antibody fragments with renal enzyme-cleavable linkage for low renal radioactivity levels
Re]Organorhenium-labeled antibody fragments with renal enzyme-cleavable linkage for low renal radioactivity levelsUehara, Tomoya*; Koike, Miho*; Nakata, Hideo*; Hanaoka, Hirofumi*; Iida, Yasuhiko*; Hashimoto, Kazuyuki; Akizawa, Hiromichi*; Endo, Keigo*; Arano, Yasushi*
Bioconjugate Chemistry, 18(1), p.190 - 198, 2007/01
Times Cited Count:20 Percentile:55.24(Biochemical Research Methods)Renal localization of radiolabeled antibody fragments constitutes a problem in targeted imaging and radiotherapy. To estimate the applicability of the molecular design to metallic radionuclides, [ Re]tricarbonyl(cyclopentadienylcarbonate)rhenium ([
Re]tricarbonyl(cyclopentadienylcarbonate)rhenium ([ Re]CpTR-COOH) was conjugated with maleoyl-glycyl-lysine to prepare [
Re]CpTR-COOH) was conjugated with maleoyl-glycyl-lysine to prepare [ Re]CpTR-GK. The cleavage of the glycyl-lysine linkage of the compound generates a glycine conjugate of [
Re]CpTR-GK. The cleavage of the glycyl-lysine linkage of the compound generates a glycine conjugate of [ Re]CpTR-Gly. [
Re]CpTR-Gly. [ Re]CpTR-GK was conjugated to thiolated Fab fragments to prepare [
Re]CpTR-GK was conjugated to thiolated Fab fragments to prepare [ Re]CpTR-GK-Fab. The biodistribution of radioactivity after injection of [
Re]CpTR-GK-Fab. The biodistribution of radioactivity after injection of [ Re]CpTR-GK-Fab was compared with that of [
Re]CpTR-GK-Fab was compared with that of [ Re]CpTR-Fab. [
Re]CpTR-Fab. [ Re]CpTR-GK-Fab exhibited significantly lower renal radioactivity levels than did [
Re]CpTR-GK-Fab exhibited significantly lower renal radioactivity levels than did [ Re]CpTR-Fab. The analysis of urine samples collected for 6 h postinjection of [
Re]CpTR-Fab. The analysis of urine samples collected for 6 h postinjection of [ Re]CpTR-GK-Fab showed that [
Re]CpTR-GK-Fab showed that [ Re]CpTR-Gly was the major radiometabolite. In tumor-bearing mice, [
Re]CpTR-Gly was the major radiometabolite. In tumor-bearing mice, [ Re]CpTR-GK-Fab significantly reduced renal radioactivity levels without impairing the radioactivity levels in tumor. These findings indicate that the molecular design of radioparmaceuticals labeled with metallic radionuclides can be useful by using a radiometal chelate of high inertness and by designing a radiometabolite of high urinary excretion when released from antibody fragments following cleavage of a glycyl-lysine linkage. This study also indicates that a change in chemical structure of a radiolabel attached to a glycyl-lysine linkage significantly affected enzymes involved in the hydrolysis reaction.
Re]CpTR-GK-Fab significantly reduced renal radioactivity levels without impairing the radioactivity levels in tumor. These findings indicate that the molecular design of radioparmaceuticals labeled with metallic radionuclides can be useful by using a radiometal chelate of high inertness and by designing a radiometabolite of high urinary excretion when released from antibody fragments following cleavage of a glycyl-lysine linkage. This study also indicates that a change in chemical structure of a radiolabel attached to a glycyl-lysine linkage significantly affected enzymes involved in the hydrolysis reaction.
 Re chelate-conjugated bisphosphonate for the development of new radiopharmaceuticals for bones
Re chelate-conjugated bisphosphonate for the development of new radiopharmaceuticals for bonesUehara, Tomoya*; Jin, Z. L.*; Ogawa, Kazuma*; Akizawa, Hiromichi*; Hashimoto, Kazuyuki; Nakayama, Morio*; Arano, Yasushi*
Nuclear Medicine and Biology, 34(1), p.79 - 87, 2007/01
Times Cited Count:23 Percentile:56.61(Radiology, Nuclear Medicine & Medical Imaging)In this study, a key factor affecting the pharmacokinetics of a chelate-conjugated BP was investigated to estimate the validity and the applicability of molecular design. Chemically inert and well-characterized [ Re]CpTR-Gly was conjugated with 3-amino-1-hydroxypropylidene-1,1-bisphosphonate and purified by HPLC to prepare [
Re]CpTR-Gly was conjugated with 3-amino-1-hydroxypropylidene-1,1-bisphosphonate and purified by HPLC to prepare [ Re]CpTR-Gly-APD. Plasma stability, plasma protein binding, hydroxyapatite (HA) binding and the pharmacokinetics of [
Re]CpTR-Gly-APD. Plasma stability, plasma protein binding, hydroxyapatite (HA) binding and the pharmacokinetics of [ Re]CpTR-Gly-APD were compared with those of
Re]CpTR-Gly-APD were compared with those of  Re 1-hydroxyethylidene-1,1-diphosphonate (HEDP). The HPLC-purified [
Re 1-hydroxyethylidene-1,1-diphosphonate (HEDP). The HPLC-purified [ Re]CpTR-Gly-APD showed higher plasma stability, higher HA binding, higher bone accumulation and lower plasma protein binding than did
Re]CpTR-Gly-APD showed higher plasma stability, higher HA binding, higher bone accumulation and lower plasma protein binding than did  Re -HEDP. Although
Re -HEDP. Although  Re -HEDP possessed HA binding and bone accumulation similar to those of [
Re -HEDP possessed HA binding and bone accumulation similar to those of [ Re]CpTR-Gly-APD, the specific activity of
Re]CpTR-Gly-APD, the specific activity of  Re -labeled BPs was found to play a crucial role in bone accumulation and blood clearance. Thus, the molecular design of chelate-conjugated BP would be useful for the development of bone-seeking radiopharmaceuticals with a variety of radionuclides by selecting chelating molecules that provide high specific activities.
Re -labeled BPs was found to play a crucial role in bone accumulation and blood clearance. Thus, the molecular design of chelate-conjugated BP would be useful for the development of bone-seeking radiopharmaceuticals with a variety of radionuclides by selecting chelating molecules that provide high specific activities.
 Tc and
Tc and  Re
ReKiyota, Sachiko*; Uehara, Tomoya*; Ishii, Daisuke*; Akizawa, Hiromichi*; Hashimoto, Kazuyuki; Arano, Yasushi*
no journal, ,
no abstracts in English
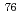 Br-labeled antibody fragments for reduction of non-target radioactivity
Br-labeled antibody fragments for reduction of non-target radioactivityHanaoka, Hirofumi*; Watanabe, Shigeki; Watanabe, Satoshi; Ohshima, Yasuhiro; Uehara, Tomoya*; Akizawa, Hiromichi*; Iida, Yasuhiko*; Ishioka, Noriko; Arano, Yasushi*; Endo, Keigo*
no journal, ,
no abstracts in English