Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Takagi, Yasuhiko*; Nakamura, Tomoki*; Hiroi, Takahiro*; Matsuoka, Moe*; et al.
Nature Astronomy (Internet), 5(3), p.246 - 250, 2021/03
Times Cited Count:58 Percentile:96.23(Astronomy & Astrophysics)Here we report observations of Ryugu's subsurface material by the Near-Infrared Spectrometer (NIRS3) on the Hayabusa2 spacecraft. Reflectance spectra of excavated material exhibit a hydroxyl (OH) absorption feature that is slightly stronger and peak-shifted compared with that observed for the surface, indicating that space weathering and/or radiative heating have caused subtle spectral changes in the uppermost surface. However, the strength and shape of the OH feature still suggests that the subsurface material experienced heating above 300  C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200
C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200  C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Arai, Takehiko*; Nakauchi, Yusuke*; Nakamura, Tomoki*; Matsuoka, Moe*; et al.
Science, 364(6437), p.272 - 275, 2019/04
Times Cited Count:298 Percentile:99.68(Multidisciplinary Sciences)The near-Earth asteroid 162173 Ryugu, the target of Hayabusa2 sample return mission, is believed to be a primitive carbonaceous object. The Near Infrared Spectrometer (NIRS3) on Hayabusa2 acquired reflectance spectra of Ryugu's surface to provide direct measurements of the surface composition and geological context for the returned samples. A weak, narrow absorption feature centered at 2.72 micron was detected across the entire observed surface, indicating that hydroxyl (OH)-bearing minerals are ubiquitous there. The intensity of the OH feature and low albedo are similar to thermally- and/or shock-metamorphosed carbonaceous chondrite meteorites. There are few variations in the OH-band position, consistent with Ryugu being a compositionally homogeneous rubble-pile object generated from impact fragments of an undifferentiated aqueously altered parent body.
Tsujimoto, Kazufumi; Arai, Yasuo; Minato, Kazuo
Nihon Genshiryoku Gakkai-Shi ATOMO , 59(11), p.644 - 648, 2017/11
, 59(11), p.644 - 648, 2017/11
no abstracts in English
Komeda, Masao; Arai, Masaji; Tamai, Kazuo*; Kawasaki, Kozo*
Applied Radiation and Isotopes, 113, p.60 - 65, 2016/07
Times Cited Count:2 Percentile:17.78(Chemistry, Inorganic & Nuclear)We designed and fabricated a new type holder that achieved uniform doping under long-term use at JRR-3. The new type holder uses an aluminum alloy and B C particles as filter materials to ensure uniform vertical flux distribution. Although the amount of filter material decreases with long-term use, it was found that the doping distribution did not change by much until 800 h. The lifetime of the new type holder (of the order of hundreds of hours) depends mainly on the amount of trapped radioactive isotopes. This indicates that the decrease in filtering ability over the filter's lifetime is not significant. The filtering ability remains stable for a long time, and the difference in the vertical doping distribution is 1.08 at 1600 h and 1.18 at 4000 h. The irradiation efficiency is expected to increase by 1.7 times when using the new type holder.
C particles as filter materials to ensure uniform vertical flux distribution. Although the amount of filter material decreases with long-term use, it was found that the doping distribution did not change by much until 800 h. The lifetime of the new type holder (of the order of hundreds of hours) depends mainly on the amount of trapped radioactive isotopes. This indicates that the decrease in filtering ability over the filter's lifetime is not significant. The filtering ability remains stable for a long time, and the difference in the vertical doping distribution is 1.08 at 1600 h and 1.18 at 4000 h. The irradiation efficiency is expected to increase by 1.7 times when using the new type holder.
Takino, Kazuo; Arai, Masaji; Murayama, Yoji
Proceedings of International Topical Meeting on Research Reactor Fuel Management and Meeting of the International Group on Reactor Research (RRFM/IGORR 2016) (Internet), p.667 - 676, 2016/03
Our working group has started to investigate basic concepts of the new research reactor which foresaw twenty years later. The aim of this project is to build up the design of new multipurpose research reactor which is constructed instead of JRR-3 for utilization of the neutron beam, irradiation, training and so on.
Mukai, Kazuo; Arai, Masanobu; Ito, Kazuhiro; Okawachi, Yasushi
Nihon Genshiryoku Gakkai-Shi ATOMO , 56(9), p.554 - 560, 2014/09
, 56(9), p.554 - 560, 2014/09
no abstracts in English
Saegusa, Jun; Kurikami, Hiroshi; Yasuda, Ryo; Kurihara, Kazuo; Arai, Shigeki; Kuroki, Ryota; Matsuhashi, Shimpei; Ozawa, Takashi; Goto, Hiroaki; Takano, Takao; et al.
Health Physics, 104(3), p.243 - 250, 2013/03
Times Cited Count:3 Percentile:24.26(Environmental Sciences)After the Nuclear accident on March 2011, water discharge from many outdoor swimming pools in the Fukushima prefecture was suspended out of concern that radiocesium in the pool water would flow into farmlands. We have reviewed the existing flocculation method for decontaminating pool water and established a practical decontamination method by demonstrating the process at several pools in the Fukushima prefecture.
 1-tetrahydrocannabinolic acid (THCA) synthase, the enzyme controlling the psychoactivity of
1-tetrahydrocannabinolic acid (THCA) synthase, the enzyme controlling the psychoactivity of 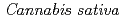
Shoyama, Yoshinari*; Tamada, Taro; Kurihara, Kazuo; Takeuchi, Ayako*; Taura, Futoshi*; Arai, Shigeki; Blaber, M.*; Shoyama, Yukihiro*; Morimoto, Satoshi*; Kuroki, Ryota
Journal of Molecular Biology, 423(1), p.96 - 105, 2012/10
Times Cited Count:90 Percentile:90.02(Biochemistry & Molecular Biology) 1-tetrahydrocannabinolic acid (THCA) synthase catalyzes the oxidative cyclization of cannabigerolic acid (CBGA) into THCA, the precursor of the primary psychoactive agent
1-tetrahydrocannabinolic acid (THCA) synthase catalyzes the oxidative cyclization of cannabigerolic acid (CBGA) into THCA, the precursor of the primary psychoactive agent  1-tetrahydrocannabinol in
1-tetrahydrocannabinol in 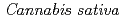 . The structure-function relationship of THCA synthase was investigated by X-ray structure determination (2.75
. The structure-function relationship of THCA synthase was investigated by X-ray structure determination (2.75  resolution) and mutational analysis. Specific amino acid residues were identified in the active site of THCA synthase that are involved in the oxidative cyclization of the CBGA substrate.
resolution) and mutational analysis. Specific amino acid residues were identified in the active site of THCA synthase that are involved in the oxidative cyclization of the CBGA substrate.
Nakamura, Kazuo*; Jiang, Y.*; Liu, X.*; Mitarai, Osamu*; Kurihara, Kenichi; Kawamata, Yoichi; Sueoka, Michiharu; Hasegawa, Makoto*; Tokunaga, Kazutoshi*; Zushi, Hideki*; et al.
Fusion Engineering and Design, 86(6-8), p.1080 - 1084, 2011/10
Times Cited Count:5 Percentile:36.88(Nuclear Science & Technology)Inamura, Yasuhiro; So, J.-Y.*; Nakajima, Kenji; Suzuki, Jiro*; Nakatani, Takeshi; Kajimoto, Ryoichi; Otomo, Toshiya*; Moon, M.-K.*; Lee, C.-H.*; Yasu, Yoshiji*; et al.
JAEA-Technology 2010-047, 74 Pages, 2011/02
This report summarizes the two-year (2007-2009) activities of Korea-Japan collaboration of chopper software development. Here we have described the background of the collaboration and the main part of our work. We also discussed briefly a future plan of our collaboration starting in 2010. Some of detailed description on the management of the collaboration as well as related information is given in appendix.
Oigawa, Hiroyuki; Minato, Kazuo; Morita, Yasuji; Kimura, Takaumi; Arai, Yasuo; Tsujimoto, Kazufumi; Nishihara, Kenji
Proceedings of 11th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (Internet), p.123 - 132, 2010/11
After the first check and review on the partitioning and transmutation (PT) technology by the Japan Atomic Energy Commission (JAEC) in 2000, significant progress was made in respective research areas of the partitioning, fuel fabrication, transmutation and fuel recycling in Japan. The second check and review on the PT technology was made by the JAEC in 2008-2009. The final report issued in April, 2009, mentions that the significance of the PT technology is in three points: reduction of the potential hazard, mitigation of the requirement for geological repository site, and enhancement of the options in the design of the whole system of waste disposal. The current technology levels of the PT for both FBR and ADS were evaluated. The PT technology in general is still in the basic research because of the lack of experimental data for minor actinides (MA). It was, therefore, strongly recommended to accumulate the experimental data for MA as a common basis for both FBR and ADS.
Nishi, Tsuyoshi; Takano, Masahide; Ito, Akinori; Miyata, Seiichi; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
IOP Conference Series; Materials Science and Engineering, 9, p.012017_1 - 012017_8, 2010/05
Times Cited Count:2 Percentile:70.08(Chemistry, Inorganic & Nuclear)The thermal diffusivities and heat capacities of transuranium nitride solid solutions, (Np,Am)N and (Pu,Am)N, were measured by using a laser flash method and a drop calorimetry, respectively. The thermal conductivities of these samples were determined from the measured thermal diffusivities, heat capacities and bulk densities. The thermal conductivities of (Np,Am)N and (Pu,Am)N increased with temperature over the temperature range investigated. The increases in the thermal conductivities were probably due to the increase of electrical components. In addition, the thermal conductivities of (Np,Am)N and (Pu,Am)N decreased with increasing Am contents. It could be considered that the decreases in the thermal conductivities correspond to the lowering of electronic contribution.
Nishi, Tsuyoshi; Takano, Masahide; Ito, Akinori; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), 8 Pages, 2010/00
Neptunium nitride (NpN) and americium nitride (AmN) were prepared by carbothermic reduction of the respective dioxides. The thermal diffusivities of NpN and AmN were measured by using a laser flash method. The heat capacities of NpN and AmN were determined with the drop calorimetry. The thermal diffusivity of NpN tends to remain unchanged with increasing temperature in the temperature range from 473 to 1473 K, and that of AmN tends to slightly decrease with increasing temperature in the same temperature range. The heat capacity of NpN obtained was close to those of UN and PuN, while that of AmN was slightly smaller than those of UN, NpN and PuN. The thermal conductivities of NpN and AmN were determined from the measured thermal diffusivities, heat capacities and bulk densities. It was found that the thermal conductivities of NpN and AmN slightly increased with temperature in the temperature range investigated.
Takano, Masahide; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), 7 Pages, 2010/00
As the research activities concerning the nitride fuel for the transmutation of minor actinides, the lattice thermal expansions of the transuranium nitrides were investigated in order to build the material database. The lattice parameters of NpN, PuN and AmN were measured by the high temperature X-ray diffraction method from room temperature to 1478 K. Their linear thermal expansions were determined as a function of temperature. The average thermal expansion coefficients over the temperature range of 293 to 1273 K for these nitrides were 8.8, 11.1 and 11.2 10
10 K
K , respectively. Further, the thermal expansions of the nitride solid solution samples, (Np,Am)N, (Pu,Am)N, (Np,Pu,Am,Cm)N and (Pu,Am,Zr)N, were measured to investigate the composition dependence. It was confirmed that the average thermal expansion coefficients for these solid solution samples could be approximated by the linear mixture rule within the error of 2
, respectively. Further, the thermal expansions of the nitride solid solution samples, (Np,Am)N, (Pu,Am)N, (Np,Pu,Am,Cm)N and (Pu,Am,Zr)N, were measured to investigate the composition dependence. It was confirmed that the average thermal expansion coefficients for these solid solution samples could be approximated by the linear mixture rule within the error of 2 3%.
3%.
Hayashi, Hirokazu; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), 9 Pages, 2010/00
R&D on the transmutation of long-lived minor actinides (MA) by the accelerator-driven system (ADS) using nitride fuels is underway at JAEA. In regard to reprocessing technology, pyrochemical process has several advantages in case of treating spent fuel with large decay heat and fast neutron emission, and recovering highly enriched 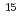 N. In the pyrochemical reprocessing, plutonium and MA are dissolved in LiCl-KCl eutectic melts and selectively recovered into liquid cadmium cathode by molten salt electrorefining. The electrochemical behavior in LiCl-KCl eutectic melts and the subsequent nitride formation behavior of plutonium and MA recovered in liquid Cd cathode are investigated. Recent results on the behavior of americium (Am) in the pyrochemical process for the treatment of spent nitride fuel, which include preparation of AmCl
N. In the pyrochemical reprocessing, plutonium and MA are dissolved in LiCl-KCl eutectic melts and selectively recovered into liquid cadmium cathode by molten salt electrorefining. The electrochemical behavior in LiCl-KCl eutectic melts and the subsequent nitride formation behavior of plutonium and MA recovered in liquid Cd cathode are investigated. Recent results on the behavior of americium (Am) in the pyrochemical process for the treatment of spent nitride fuel, which include preparation of AmCl and electrochemical behavior of Am in LiCl-KCl eutectic melts, are presented.
and electrochemical behavior of Am in LiCl-KCl eutectic melts, are presented.
Arai, Yasuo; Akabori, Mitsuo; Minato, Kazuo; Uno, Masayoshi*
Proceedings of 10th OECD/NEA Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (CD-ROM), p.189 - 197, 2010/00
The progress of R&D on nitride fuel and pyrochemical process for transmutation of minor actinides are described. Nitride fuel was prepared by carbothermic reduction and characterized by X-ray diffraction and chemical analyses. In addition to minor actinide nitrides, burnup simulated nitrides and nitrides with inert diluent materials were prepared, and the thermal properties such as thermal conductivity were obtained. With regard to pyrochemical process, the electrode behavior of the above nitrides in the molten salt was clarified, and nitride power synthesized from actinides recovered in liquid cadmium cathode was used for nitride pellet preparation. Further, the irradiation tests of nitride fuel carried out in JAEA are introduced.
Hayashi, Hirokazu; Shibata, Hiroki; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.1166 - 1173, 2009/09
R&D on the transmutation of long-lived minor actinides (MA) by the accelerator-driven system (ADS) using nitride fuels is underway at JAEA. In regard to reprocessing technology, pyrochemical process has several advantages in case of treating spent fuel with large decay heat and fast neutron emission, and recovering highly enriched 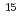 N. In the pyrochemical reprocessing, plutonium and MA are dissolved in LiCl-KCl eutectic melts and selectively recovered into liquid cadmium (Cd) cathode by molten salt electrorefining. The electrochemical behavior in LiCl-KCl eutectic melts and the subsequent nitride formation behavior of plutonium and MA recovered in liquid Cd cathode are investigated. Electrochemical study of americium (Am) on electrolyses of AmN in LiCl-KCl eutectic melts and nitride formation of Am recovered in the liquid Cd cathode are presented. Electrochemical behavior of Am on anodic dissolution of AmN and recovery of Am into a liquid Cd cathode by electrolyses in LiCl-KCl eutectic melts was investigated by transient electrochemical techniques. Am was recovered as Am-Cd alloy in the liquid Cd cathode, in which AmCd
N. In the pyrochemical reprocessing, plutonium and MA are dissolved in LiCl-KCl eutectic melts and selectively recovered into liquid cadmium (Cd) cathode by molten salt electrorefining. The electrochemical behavior in LiCl-KCl eutectic melts and the subsequent nitride formation behavior of plutonium and MA recovered in liquid Cd cathode are investigated. Electrochemical study of americium (Am) on electrolyses of AmN in LiCl-KCl eutectic melts and nitride formation of Am recovered in the liquid Cd cathode are presented. Electrochemical behavior of Am on anodic dissolution of AmN and recovery of Am into a liquid Cd cathode by electrolyses in LiCl-KCl eutectic melts was investigated by transient electrochemical techniques. Am was recovered as Am-Cd alloy in the liquid Cd cathode, in which AmCd type phase was identified besides Cd phase. The recovered Am was converted to AmN by the nitridation-distillation combined method. These results suggest that the pyrochemical process developed for the nitride fuel cycle is applicable for the nitride fuels containing Am, which is to be used for the transmutation of MA.
type phase was identified besides Cd phase. The recovered Am was converted to AmN by the nitridation-distillation combined method. These results suggest that the pyrochemical process developed for the nitride fuel cycle is applicable for the nitride fuels containing Am, which is to be used for the transmutation of MA.
Minato, Kazuo; Morita, Yasuji; Kimura, Takaumi; Oigawa, Hiroyuki; Arai, Yasuo; Sasa, Toshinobu
Proceedings of International Conference on Advanced Nuclear Fuel Cycle; Sustainable Options & Industrial Perspectives (Global 2009) (CD-ROM), p.504 - 512, 2009/09
In JAEA, research and development (R&D) activities for partitioning and transmutation (P&T) have been promoted under the OMEGA program for 20 years. The first review of these activities was performed by the Japan Atomic Energy Commission in 2000. In accordance with the results of the review, JAEA is conducting the R&D on the P&T technology based on the "Double-strata Fuel Cycle concept" as well as that based on the commercial fast reactor cycle. The "Double-strata Fuel Cycle concept" consists of the partitioning process of the high-level waste (HLW) and the dedicated transmutation cycle using the accelerator-driven system (ADS) with the minor actinide (MA) nitride fuel. The benefit of P&T technology in terms of the waste management is also discussed. The second check and review on P&T was made in September 2008 - March 2009. The review results and recommendations for future R&D are introduced.
Hayashi, Hirokazu; Shibata, Hiroki; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.673 - 676, 2009/08
Times Cited Count:7 Percentile:16.58(Electrochemistry)R&D of the nitride fuel cycle technology is underway at Japan Atomic Energy Agency (JAEA). Behavior of americium (Am) in the pyrochemical process, which includes anodic dissolution of AmN and recovery of Am into a liquid cadmium (Cd) cathode by electrolyses in LiCl-KCl eutectic melts, and nitride formation of Am recovered in liquid Cd, is presented.
Minato, Kazuo; Takano, Masahide; Otobe, Haruyoshi; Nishi, Tsuyoshi; Akabori, Mitsuo; Arai, Yasuo
Journal of Nuclear Materials, 389(1), p.23 - 28, 2009/05
Times Cited Count:15 Percentile:67.67(Materials Science, Multidisciplinary)Burning or transmutation of minor actinides (MA: Np, Am, Cm) that are classified as the high-level radioactive waste in the current nuclear fuel cycle is an option for the advanced nuclear fuel cycle. Although the thermochemical and thermophysical properties of minor actinide compounds are essential for the design of MA-bearing fuels and analysis of their behavior, the experimental data on minor actinide compounds are limited. To support the research and development of the MA-bearing fuels, the property measurements were carried out on minor actinide nitrides and oxides. The lattice parameters and their thermal expansions were measured by high-temperature X-ray diffractometry. The specific heat capacities were measured by drop calorimetry and the thermal diffusivities by laser-flash method. The thermal conductivities were determined by the specific heat capacities, thermal diffusivities and densities. The oxygen potentials were measured by electromotive force method.