Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Arai, Yoichi; Watanabe, So; Watanabe, Masayuki; Arai, Tsuyoshi*; Katsuki, Kenta*; Agou, Tomohiro*; Fujikawa, Hisaharu*; Takeda, Keisuke*; Fukumoto, Hiroki*; Hoshina, Hiroyuki*; et al.
Nuclear Instruments and Methods in Physics Research B, 554, p.165448_1 - 165448_10, 2024/09
Times Cited Count:0 Percentile:0.00(Instruments & Instrumentation)Arai, Yoichi; Hasegawa, Kenta; Watanabe, So; Watanabe, Masayuki; Minowa, Kazuki*; Matsuura, Haruaki*; Hagura, Naoto*; Katsuki, Kenta*; Arai, Tsuyoshi*; Konishi, Yasuhiro*
Journal of Radioanalytical and Nuclear Chemistry, 333(7), p.3585 - 3593, 2024/07
Times Cited Count:1 Percentile:23.64(Chemistry, Analytical)Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 40(2), p.347 - 352, 2024/02
Times Cited Count:2 Percentile:23.37(Chemistry, Analytical)The Eu(III) distribution mechanism in single extractant-impregnated polymer-layered silica particle in a complex solution containing multiple lanthanide ions was investigated using fluorescence microspectroscopy. The rate-determining step was the reaction of Eu(III) with the two extractant molecules. The obtained mechanism and rate constants agreed with those of the single-ion distribution system, in which Eu(III) was distributed to the particles in the Eu(III) solution.
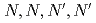 -tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degree
-tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degreeMiyagawa, Akihisa*; Takahashi, Takumi*; Kuzure, Yoshiaki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 39(11), p.1929 - 1936, 2023/11
Times Cited Count:1 Percentile:10.71(Chemistry, Analytical)In this study, we revealed the Eu(III) distribution in a single diglycolamide-derivative extractant (TODGA)-impregnated polymer-coated silica particle. The reaction of Eu(III) with two TODGA molecules in the polymer layer was the rate-limiting process, as evidenced by the absence of any correlation between the rate constants (k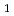 and k
and k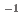 ) and concentrations of Eu(III) and HNO
) and concentrations of Eu(III) and HNO .
.
Watanabe, So; Takahatake, Yoko; Ogi, Hiromichi*; Osugi, Takeshi; Taniguchi, Takumi; Sato, Junya; Arai, Tsuyoshi*; Kajinami, Akihiko*
Journal of Nuclear Materials, 585, p.154610_1 - 154610_6, 2023/11
Times Cited Count:1 Percentile:0.00(Materials Science, Multidisciplinary)Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 96(9), p.1019 - 1025, 2023/09
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)In the present study, we have elucidated the mass transfer mechanism of Eu(III) and Sm(III) in the solution with these ions in single nitrilotriacetamide (NTA) extractant-impregnated polymer-coated silica particle. The rate-limiting process of mass transfer was the reaction process of ions with NTA molecules, in which the NO
 ions were not involved, which was consistent with that obtained in single ion distribution system.
ions were not involved, which was consistent with that obtained in single ion distribution system.
Miyagawa, Akihisa*; Hayashi, Naoki*; Kuzure, Yoshiaki*; Takahashi, Takumi*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; et al.
Bulletin of the Chemical Society of Japan, 96(7), p.671 - 676, 2023/07
Times Cited Count:8 Percentile:61.41(Chemistry, Multidisciplinary)We investigated the distribution mechanism of Eu(III) in a single polymer-coated silica particle including nitrilotriacetamide (NTA) extractants known as HONTA and TOD2EHNTA. The present study provides a valuable approach for the evaluation and enhancement of the functionality of "single extractant-impregnated polymer-coated silica particle".
Takahatake, Yoko; Watanabe, So; Arai, Tsuyoshi*; Sato, Takahiro*; Shibata, Atsuhiro
Applied Radiation and Isotopes, 196, p.110783_1 - 110783_5, 2023/06
Times Cited Count:2 Percentile:43.92(Chemistry, Inorganic & Nuclear)Akuzawa, Tadashi*; Kim, S.-Y.*; Kubota, Masahiko*; Wu, H.*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 331(12), p.5851 - 5858, 2022/12
Times Cited Count:5 Percentile:60.29(Chemistry, Analytical)Sano, Yuichi; Sakamoto, Atsushi; Miyazaki, Yasunori; Watanabe, So; Morita, Keisuke; Emori, Tatsuya; Ban, Yasutoshi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; et al.
Proceedings of International Conference on Nuclear Fuel Cycle; Sustainable Energy Beyond the Pandemic (GLOBAL 2022) (Internet), 4 Pages, 2022/07
We developed a hybrid MA(III) recovery process combining MA(III)+Ln(III) co-recovery flowsheet by solvent extraction with TBP and MA(III)/Ln(III) separation flowsheet by simulated moving bed chromatography using HONTA impregnated adsorbents with large particle size porous silica support.
Miyagawa, Akihisa*; Takeuchi, Masayuki; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 95(4), p.566 - 568, 2022/04
Times Cited Count:3 Percentile:20.54(Chemistry, Multidisciplinary)We demonstrate that pKa of extractant impregnated in a polymer phase varies with the cross-linking degree and the coexistence of other extractants, which induces a change in the hydrophobicity of the polymer phase. The results presented herein will be beneficial for the development of novel solid-extraction adsorbents.
 -P for an advanced reprocessing
-P for an advanced reprocessingHoriuchi, Yusuke; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Kida, Fukuka*; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 330(1), p.237 - 244, 2021/10
Times Cited Count:11 Percentile:77.39(Chemistry, Analytical)Applicability of tetra2-ehylhexyl diglycolamide (TEHDGA) impregnated adsorbent for minor actinide (MA) recovery from high level liquid waste (HLLW) in extraction chromatography technology was investigated through batch-wise adsorption and column separation experiments. Distribution ratio of representative fission product elements were obtained by the batch-wise experiments, and TEHDGA adsorbent was shown to be preferable to TODGA adsorbent for decontamination of several species. All Ln(III) supplied into the TEHDGA adsorbent packed column was properly eluted from the column, and the applicability of the adsorbent was successfully showed by this study.
Arai, Yoichi; Watanabe, So; Ono, Shimpei; Nomura, Kazunori; Nakamura, Fumiya*; Arai, Tsuyoshi*; Seko, Noriaki*; Hoshina, Hiroyuki*; Hagura, Naoto*; Kubota, Toshio*
Nuclear Instruments and Methods in Physics Research B, 477, p.54 - 59, 2020/08
Times Cited Count:7 Percentile:55.09(Instruments & Instrumentation)Arai, Yoichi; Watanabe, So; Ono, Shimpei; Nomura, Kazunori; Nakamura, Fumiya*; Arai, Tsuyoshi*; Seko, Noriaki*; Hoshina, Hiroyuki*; Kubota, Toshio*
QST-M-23; QST Takasaki Annual Report 2018, P. 59, 2020/03
Watanabe, So; Senzaki, Tatsuya; Shibata, Atsuhiro; Nomura, Kazunori; Takeuchi, Masayuki; Nakatani, Kiyoharu*; Matsuura, Haruaki*; Horiuchi, Yusuke*; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 322(3), p.1273 - 1277, 2019/12
Times Cited Count:6 Percentile:47.17(Chemistry, Analytical)Watanabe, So; Ogi, Hiromichi*; Arai, Yoichi; Aihara, Haruka; Takahatake, Yoko; Shibata, Atsuhiro; Nomura, Kazunori; Kamiya, Yuichi*; Asanuma, Noriko*; Matsuura, Haruaki*; et al.
Progress in Nuclear Energy, 117, p.103090_1 - 103090_8, 2019/11
Times Cited Count:14 Percentile:77.49(Nuclear Science & Technology)Otaka, Toshiki*; Sato, Tatsumi*; Ono, Shimpei; Nagoshi, Kohei; Abe, Ryoji*; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Nakatani, Kiyoharu*
Analytical Sciences, 35(10), p.1129 - 1133, 2019/10
Times Cited Count:9 Percentile:33.12(Chemistry, Analytical)Watanabe, So; Katai, Yuya*; Matsuura, Haruaki*; Kada, Wataru*; Koka, Masashi*; Sato, Takahiro*; Arai, Tsuyoshi*
Nuclear Instruments and Methods in Physics Research B, 450, p.61 - 65, 2019/07
Times Cited Count:4 Percentile:35.13(Instruments & Instrumentation) -P adsorbent on extraction chromatography process
-P adsorbent on extraction chromatography processWatanabe, So; Sano, Yuichi; Sanda, Shuhei*; Sakurai, Shota*; Arai, Tsuyoshi*
Nihon Ion Kokan Gakkai-Shi, 30(1), p.8 - 16, 2019/01
Arai, Yoichi; Watanabe, So; Ono, Shimpei; Nakamura, Masahiro; Shibata, Atsuhiro; Nakamura, Fumiya*; Arai, Tsuyoshi*; Seko, Noriaki*; Hoshina, Hiroyuki*; Hagura, Naoto*; et al.
International Journal of PIXE, 29(1&2), p.17 - 31, 2019/00
The spent PUREX solvent containing U and Pu is generated from the reprocessing process of spent nuclear fuel. The nuclear material removal is important for the safe storage or disposal of the spent solvent. Our previous study revealed that the adsorbent with the iminodiacetic acid (IDA) functional group is one of the most promising materials for designing the nuclear material recovery process. Accordingly, an IDA-type adsorbent was synthesized by using graft polymerization technology or a chemical reaction to improve the adsorption rate and capacity. The synthesized IDA-type adsorbent was characterized by micro particle-induced X-ray emission (PIXE) and extended X-ray absorption fine structure (EXAFS) analyses. The micro-PIXE analysis revealed that Zr was adsorbed on the whole synthesized adsorbents and quantified the microamount of adsorbed Zr. Moreover, EXAFS suggested that Zr in the aqueous solution and solvent can be trapped by the IDA group with different mechanisms.