Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Nakajima, Kenji; Kawamura, Seiko; Kikuchi, Tatsuya; Fujiwara, Satoru
Biochemistry and Biophysics Reports (Internet), 6, p.220 - 225, 2016/07
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Nakajima, Kenji; Kawamura, Seiko; Kikuchi, Tatsuya; Fujiwara, Satoru
Biochemical and Biophysical Research Communications, 459(3), p.493 - 497, 2015/04
Times Cited Count:4 Percentile:12.91(Biochemistry & Molecular Biology)Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
Biophysics, 9, p.99 - 106, 2013/07
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
no journal, ,
The structural and dynamic properties of hydration water around F-actin and myosin S1 were investigated using small-angle neutron/X-ray scattering and quasi-elastic neutron scattering. S1 was shown to have typical hydration water, which has 10-15% higher average density with lower mobility than bulk water. On the other hand, F-actin was shown to have hydration water with unusual properties: the average density of the hydration water is at least 19% higher than that of bulk water and mobility is close to that of bulk water. These results indicate the diversity of hydration shell around proteins in terms of both structural and dynamic properties. The unusual hydration water around F-actin may be related to the suggested existence of "hyper-mobile water" around F-actin.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
no journal, ,
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
no journal, ,
In this study, we investigated the dynamics of the hydration water around F-actin and myosin S1, which are proteins interacting with each other to produce force in muscle contraction, by quasielastic neutron scattering (QENS). The QENS measurements were conducted on the solution samples of F-actin (150 mg/ml) and S1 (80 mg/ml) in H O and D
O and D O using the cold-neutron disk-chopper spectrometer AMATERAS in MLF/J-PARC, Japan. The spectra of hydration water were obtained by subtracting those of proteins and those of bulk water from the measured spectra of H
O using the cold-neutron disk-chopper spectrometer AMATERAS in MLF/J-PARC, Japan. The spectra of hydration water were obtained by subtracting those of proteins and those of bulk water from the measured spectra of H O samples (the spectra of proteins were obtained by subtracting those of D
O samples (the spectra of proteins were obtained by subtracting those of D O buffer from those of D
O buffer from those of D O samples, while the spectra of H
O samples, while the spectra of H O buffer was used as those of bulk water) with appropriate scaling factors. The translational diffusion coefficients(D
O buffer was used as those of bulk water) with appropriate scaling factors. The translational diffusion coefficients(D ) and the residence time (
) and the residence time (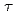
 ) were evaluated from the dependence of the half-widths at half-maximum of the Lorentzian functions fit to the spectra on the momentum transfer. In the current analysis, it was found that the D
) were evaluated from the dependence of the half-widths at half-maximum of the Lorentzian functions fit to the spectra on the momentum transfer. In the current analysis, it was found that the D value of the hydration water around S1was smaller than that of bulk water while the
value of the hydration water around S1was smaller than that of bulk water while the 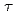
 value was larger than that of bulk water, suggesting that S1 has typical hydration water, the mobility of which is less than that of bulkwater. On the other hand, both the D
value was larger than that of bulk water, suggesting that S1 has typical hydration water, the mobility of which is less than that of bulkwater. On the other hand, both the D and the
and the 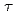
 values of the hydration water around F-actin were closer to those of bulk water, suggesting that the hydration water around F-actin has higher mobility than that around other proteins including S1. The results of a more detailed analysis will be given in the presentation.
values of the hydration water around F-actin were closer to those of bulk water, suggesting that the hydration water around F-actin has higher mobility than that around other proteins including S1. The results of a more detailed analysis will be given in the presentation.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
no journal, ,
The dynamics of F-actin and myosin S1 were studied by quasi-elastic neutron scattering (QENS). QENS measurements were conducted on D O solution samples of F-actin and S1 at 300 K using AMATERAS in J-PARC. The QENS spectra of proteins were obtained by subtracting those of D
O solution samples of F-actin and S1 at 300 K using AMATERAS in J-PARC. The QENS spectra of proteins were obtained by subtracting those of D O buffer from those of D
O buffer from those of D O samples. In the current analysis, it was found that while the correlation time of atomic motions of S1 was similar to that of other proteins studied so far, the correlation time of F-actin was shorter than S1. Furthermore, F-actin had a population of the atoms undergoing diffusive motions with larger amplitudes than S1. These results suggest that F-actin is more flexible than other proteins including S1.
O samples. In the current analysis, it was found that while the correlation time of atomic motions of S1 was similar to that of other proteins studied so far, the correlation time of F-actin was shorter than S1. Furthermore, F-actin had a population of the atoms undergoing diffusive motions with larger amplitudes than S1. These results suggest that F-actin is more flexible than other proteins including S1.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
no journal, ,
The dynamics of F-actin and myosin S1 were studied by quasi-elastic neutron scattering (QENS). QENS measurements were conducted on D2O solution samples of F-actin and S1 at 300 K using AMATERAS in J-PARC. It was found that while the correlation time of atomic motions of S1 was similar to that of other proteins studied so far, that of F-actin was shorter than S1. Furthermore, the fraction of immobile atoms were found to be larger for S1 than F-actin. These results suggest that F-actin is more flexible than other proteins including S1.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
no journal, ,
The dynamics of F-actin and myosin S1 were studied by quasi-elastic neutron scattering (QENS). QENS measurements were conducted on D2O solution samples of F-actin and S1 at 300 K using AMATERAS in J-PARC. The QENS spectra of proteins were obtained by subtracting those of D O buffer from those of D
O buffer from those of D O samples. It was found that while the correlation time of atomic motions of S1 was similar to that of other proteins studied so far, that of F-actin was shorter than S1. Furthermore, F-actin had a population of the atoms undergoing diffusive motions with larger amplitudes than S1. These results suggest that F-actin is more flexible than other proteins including S1.
O samples. It was found that while the correlation time of atomic motions of S1 was similar to that of other proteins studied so far, that of F-actin was shorter than S1. Furthermore, F-actin had a population of the atoms undergoing diffusive motions with larger amplitudes than S1. These results suggest that F-actin is more flexible than other proteins including S1.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
no journal, ,
The dynamics of F-actin and myosin S1, and their hydration water were studied by quasielastic neutron scattering (QENS). Analysis of the spectra found that a larger fraction of the atoms of F-actin undergoes the motions with the smaller residence time than S1. It was also found that the mobility of the hydration water of S1 is lower than that of bulk water, while that of the hydration water of F-actin is slightly higher than that of bulk water, evaluated by their translational diffusion coefficient and the residence time. These results suggest that the concerted action of rapidly fluctuating F-actin and its hydration water allows F-actin to explore a wide range of the conformational space, which would facilitate the binding of myosin to F-actin.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
no journal, ,
The picosecond dynamics of F-actin, myosin S1, and their hydration water were studied by quasielastic neutron scattering (QENS) at J-PARC. Analysis of the QENS spectra showed that a larger fraction of the atoms of F-actin undergoes the motions with the smaller residence time than S1. It was also found that the mobility of the hydration water of S1, which was evaluated from the translational diffusion coefficient, the residence time, and the rotational correlation time, is lower than that of bulk water, while that of the hydration water of F-actin is close to that of bulk water. These results suggest that the concerted action of rapidly fluctuating F-actin and its hydration water allows F-actin to explore a wide range of the conformational space, which would facilitate the binding of myosin to F-actin.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
no journal, ,
We studied the dynamics of F-actin, myosin S1 (S1), and their hydration water using quasi elastic neutron scattering (QENS). The analysis of the QENS spectra shows that the residence time of the atoms of F-actin is shorter than that of S1, and that the fraction of the "immobile" atoms is smaller for F-actin. These results suggest that more atoms fluctuate more rapidly in F-actin. The analysis of the spectra of their hydration water shows that the translational diffusion coefficient is smaller for S1 than F-actin. Whereas the residence time is similar between F-actin and S1 within the experimental errors, the rotational correlation time is smaller for F-actin. Furthermore, the translational diffusion coefficient and the rotational correlation time of the hydration water of F-actin are close to those of bulk water. These results imply that the concerted action between F-actin and its hydration water with high mobility allows F-actin to take quickly suitable conformations for S1 binding.